Introduction: Chronic myeloproliferative neoplasms (MPN) share the same driver mutations but their disease course and prognosis varies significantly. Deficiency of DNA damage repair (DDR) due to germline mutation is known to predispose to certain cancer types and has been implicated in the biology of MPN. The aim of our study was to evaluate the impact of germline variants in DDR genes in the natural history and outcomes of MPN patients.
Patients and Methods: 76 individuals who were diagnosed with MPN (essential thrombocytosis (ET), polycythemia vera (PV) and primary myelofibrosis (PMF)) at Johns Hopkins University Hospital were included in this study. Targeted sequencing of 63 genes implicated in myeloid malignancies was performed as part of a standard clinical evaluation. Germline variants were determined by a variant allele frequency between 40-60% in blood samples and presence in the dbSNP database. Only rare variants (minor allele frequency < 0.01) were included in this analysis. Regression analysis was used to determine the association of the presence of germline variants with disease phenotype, driver mutation, number of somatic mutations, age and sex. Cox regression and Kaplan-Meier were used to assess the implication of germline variants in the progression to MF.
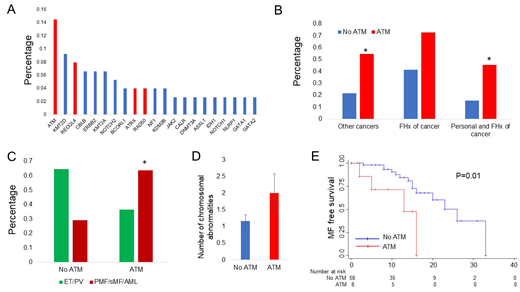
Results: Median time from diagnosis to enrollment and follow up were 6 and 11 years respectively. 22 patients (28.9%) had at least one variant in a DDR gene, with ATM the most frequent (11/76 patients, 14.5%). Other recurrently mutated DDR genes included RECQL4 (6/76 patients, 7.9%), ATRX and RAD50 (Figure 1A). Patients with an ATM germline variant had higher incidence of a second malignancy (OR 4.37, 95% CI 1.16 - 16.46, P=0.029), a non-significant trend toward positive family history of malignancy (OR 3.75, 95% CI 0.91 - 15.46, P=0.067) and higher incidence of both second malignancy and positive family history of cancer (OR 4.58, 95% CI 1.17 - 17.94, P=0.029) (Figure 1B). The presence of an ATM germline variant was associated with MF or AML as opposed to ET or PV at the time of sequencing (RRR 5.84, 95% CI 1.12 - 30.34, P=0.036) independently of driver mutation, number of additional somatic mutations, age and male sex (Figure 1C). We did not find a significant difference in the number of somatic mutations between patients with and without ATM variant, however there was a trend toward increased chromosomal abnormalities among patients with ATM variant (Figure 1D). Finally, the presence of ATM variant was associated with higher risk of MF transformation (HR 3.43, 95% CI 1.02 - 11.6, P=0.047) independently of driver mutation (JAK2 Ref, CALR - HR 0.79, 95% CI 0.21 - 2.94, P=0.73) and male sex (HR 1.4, 95% CI 0.52 - 3.76, P=0.68). Kaplan-Meier analysis confirmed that progression to MF-free survival was shorter in patients harboring an ATM variant (P=0.01) (Figure 1E).
Conclusion: The presence of a DDR gene germline variant, particularly ATM, is relatively common among patients with MPN. Patients with ATM variants had higher incidence of additional cancers and family history of malignancy, as well as an association with MF/AML phenotype and early transformation to MF. These data suggest involvement of ATM signaling in the progression of MPN, potentially via accumulation of DNA damage and genomic instability.
Figure 1. A. Frequency of germline variants in MPN patients. The graph includes the gene variants identified in at least 2 distinct patients. Of known DDR related genes (in red) ATM is the most frequent (11/76 patients, 14.5%), followed by RECQL4 (6/76 patients, 7.9%) and ATRX and RAD50. B. Personal and family history of cancer among patients with and without ATM variant. Patients with ATM germline variant have higher incidence of additional malignancy (OR 4.37, 95% CI 1.16 - 16.46, P=0.029), higher incidence of family history of malignancy (OR 3.75, 95% CI 0.91 - 15.46, P=0.067) and higher incidence of concurrent personal history of malignancy and family history of malignancy (OR 4.58, 95% CI 1.17 - 17.94, P=0.029). C. MPN subtype per ATM variant status. Patients with an ATM germline variant were more likely to have MF or AML at the time of sequencing independent of driver mutation, number of somatic mutations, age and sex. D. Patients with an ATM variant had higher number of chromosomal abnormalities. E. Kaplan-Meier analysis of progression to MF free survival(P=0.01).
Chaturvedi:Shire/Takeda: Research Funding; Alexion: Consultancy; Sanofi: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal