BACKGROUND: Ineffective erythropoiesis in thalassemia alters iron homeostasis, predisposing to systemic iron overload. Successful allogeneic hematopoietic stem cell transplantation (HSCT) in thalassemia major corrects anemia, should eliminate ineffective erythropoiesis (IE) and normalize iron homeostasis (IH). Whether gene therapy (GT) will fully correct IE and IH is not known. This cross-sectional observational study evaluated the iron status of patients with beta thalassemia following HSCT or GT, and compared them with cohorts of patients with thalassemia intermedia (TI) or transfusion-dependent thalassemia (TDT) using recently introduced biomarkers along with imaging studies and other clinical assessments to better understand and characterize IE and IH across groups.
METHODS: We evaluated a convenience sample of 29 participants with beta thalassemia (median age 25 years, IQR 21-35; females 55%; Asian 52%). Participants in the HSCT (n=6) and GT (n=10) groups were evaluated on average 116.5 and 46.9 months following cell infusion, respectively. TDT patients (n= 9) were evaluated pre-transfusion and off iron chelation for at least 7 days, and TI (n=4) were un-transfused or not transfused in >3 years. Clinical lab assessments and MRI R2*/ T2* to assess heart and liver iron burden including post-processing, were performed using local clinical protocols. ELISAs for hepcidin, erythroferrone (Erfe) and GDF-15 were performed in a blinded manner.
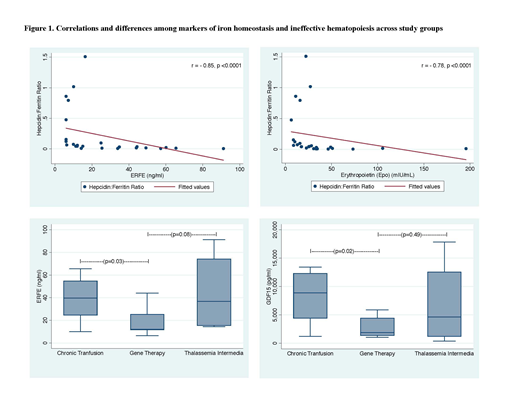
RESULTS: Median values for all IE and IH parameters tested were normal in the HSCT group, and were significantly lower than in all other groups. There were significant differences among all groups for hemoglobin (p=0.003), erythropoietin (Epo) (p=0.03), serum ferritin (SF) (p=0.01), transferrin (p=0.006), soluble transferrin receptor (sTfR) (p=0.02), serum hepcidin: serum ferritin (H:F) ratio (p=0.006), Erfe (p=0.001), GDF15 (p=0.003), and liver iron content (LIC) by MRI R2* (p=0.02). H:F ratio, a surrogate for predisposition to systemic iron loading, inversely correlated with Erfe (rs= -0.85, p<0.0001), GDF15 (rs= -0.69, p=0.0001) and liver R2* (rs= -0.66, p=0.0004). In a multivariate analysis, adjusted for gender and race, H:F ratio and Epo levels predicted Erfe and GDF15 (p=0.05 and p=0.06; p=0.01 and p=0.05), respectively. Even after excluding GT patients that are not transfusion independent (N=2), SF, Epo, sTfR and hepcidin remain abnormal in the GT group, and there were no significant differences in these parameters between GT and TDT. However, novel biomarkers of IH and IE suggested lower ineffective erythropoiesis in GT compared to TDT (median (IQR) Erfe, 12 (11.6-25.2) vs. 39.6 (24.5-54.7), p=0.03; GDF15, 1909.9 (1389-4431) vs. 8906 (4421-12331), p=0.02), respectively. Erfe and GDF15 were also lower in GT compared to TI, however these differences did not reach statistical significance. There were no differences in hepcidin, ferritin, or H:F by race, however Erfe and GDF15 were significantly lower in Asians compared to non-Asians (p=0.006 and p=0.02, respectively).
CONCLUSION: Nearly 4 years post infusion, most subjects with TDT treated with GT are transfusion independent with near normal hemoglobin, however, studies in this limited cohort using conventional measures suggest IE and IH improve, particularly when transfusion support is no longer needed, however they remain abnormal compared to HSCT recipients, who using these parameters appear to be cured. STfR did not detect differences, however GDF15 and Erfe were more sensitive assays that could demonstrate significant improvement in IE and IH with GT compared to TDT. Contribution to IE by uncorrected stem cell populations post GT cannot be determined. Transduction enhancement and other recent improvements to GT may yield different results. Longitudinal studies are needed to determine if thalassemia patients treated with GT will have ongoing IE predisposing to systemic iron overload.
Thompson:bluebird bio, Inc.: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Baxalta: Research Funding. Ganz:Intrinsic LifeSciences: Consultancy, Equity Ownership. Nemeth:Intrinsic LifeSciences: Consultancy, Equity Ownership; Silarus Therapeutics: Consultancy, Equity Ownership; Keryx: Consultancy; Ionis Pharmaceuticals: Consultancy; La Jolla Pharma: Consultancy; Protagonist: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal