Introduction: Ibrutinib, an orally and continuously administered Bruton tyrosine kinase inhibitor, allows for targeted, non-chemotherapy based treatment for chronic lymphocytic leukemia (CLL). While the initial US marketing approval for ibrutinib in CLL was for relapsed disease (February 2014), several trials have since shown favorable efficacy in the first-line setting. Ibrutinib was well tolerated in these pivotal studies, with discontinuation occurring in less than 9% at 12 months (O'Brien et al., Am J Hemato. 2019). Most early ibrutinib discontinuations in the first-line setting are due to adverse events (Tedeschi et al., EHA 2019), and optimal strategies to manage ibrutinib-related toxicities are not well defined. We hypothesized that ibrutinib is increasingly utilized for first-line treatment of CLL during routine management in the US, but that ibrutinib discontinuation rates for the real-world population are greater than those reported in clinical trials. Further, we expected to find greater odds of discontinuation in older adults and those with poor performance status, two groups underrepresented in clinical trials.
Methods: We conducted a retrospective cohort study of CLL patients treated at community oncology practices using the nationwide Flatiron Health database. The Flatiron Health database is a longitudinal, demographically and geographically diverse database derived from de-identified electronic health record data from over 280 cancer clinics in the US. All patients initiated CLL therapy in the first-line setting between March 1, 2014 and February 28, 2019. We first assessed trends in the use of first-line ibrutinib (i.e., ibrutinib with/without an anti-CD20 antibody). We then focused on patients who received ibrutinib and had at least 180 days of available follow up or had died within this period. We used multivariable logistic regression to assess the association of patient characteristics with early discontinuation (defined as stopping within 180 days of initiation). Covariates included in our model were patient age, sex, race, insurance type, ECOG performance status, year of treatment, time from CLL diagnosis to ibrutinib initiation, chromosome 17p status, and geographical region.
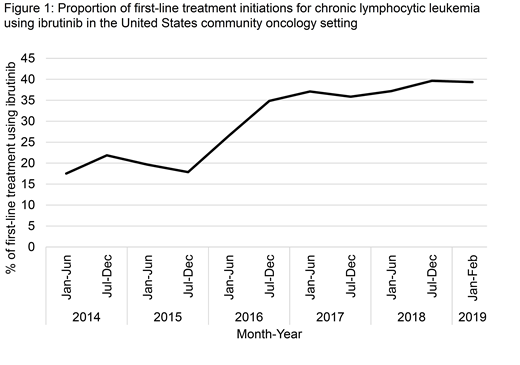
Results: We identified 5,634 individuals initiating first-line treatment for CLL, of whom 1,639 (29.1%) received an ibrutinib-based regimen. Use of ibrutinib in the first-line setting increased over time (Cochrane-Armitage test for trend, p <.001; Figure 1), making up approximately 40% of first-line CLL initiations by late 2018. Of the 1,497 individuals with adequate follow up, early discontinuation of ibrutinib occurred in 16.2%. Early discontinuation of ibrutinib was more common in individuals aged ≥80 years (26.2% vs. 9.4% for individuals 60-70 years old), those requiring treatment within 1 year of their CLL diagnosis (22.1% vs. 14.8% for those with CLL diagnosis ≥5 years prior to ibrutinib initiation), and those with ECOG performance status of 2 or greater (33.8% vs. 10.2% for ECOG = 0). On multivariable logistic regression, older age (≥80 years vs. 60-70 years, adjusted odds ratio (aOR) = 2.94, 95% confidence interval (CI) 1.79 - 4.76, p <.001) and worse performance status (ECOG ≥2 vs 0, aOR = 3.45, 95% CI 2.16-5.53, p <.001) remained independently associated with increased odds of early ibrutinib discontinuation.
Conclusion: While ibrutinib was increasingly utilized in the first-line setting for CLL within our large representative US community patient cohort, early discontinuation was more common than reported in pivotal trials. Given the complexity of the rapidly changing CLL treatment landscape, clinical decision support tools and other modern cancer care delivery approaches should be explored to improve administration of novel CLL therapies in the real-world setting.
Huntington:Genentech: Consultancy; Bayer: Consultancy, Honoraria; AbbVie: Consultancy; Celgene: Consultancy, Research Funding; DTRM Biopharm: Research Funding; Pharmacyclics: Honoraria. Barr:Merck: Consultancy; Janssen: Consultancy; AbbVie: Consultancy; Pharmacyclics LLC, an AbbVie company: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Seattle Genetics: Consultancy; Celgene: Consultancy; Genentech: Consultancy; Verastem: Consultancy; Gilead: Consultancy; Astra Zeneca: Consultancy, Research Funding. Jacobs:Genentech: Speakers Bureau; AstraZeneca: Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Research Funding, Speakers Bureau; TG Therapeutics: Honoraria, Research Funding; AbbVie: Consultancy, Speakers Bureau; JUNO: Consultancy; Gilead: Consultancy. Davidoff:PhRMA: Research Funding; Celgene: Research Funding. Gross:Johnson and Johnson: Research Funding; 21st Century Cancer: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal