Background: Autologous hematopoietic cell transplantation (AHCT) is an effective treatment to achieve deep and durable remission in multiple myeloma (MM). Typically, AHCT is offered to patients <70 years of age, although the median age of diagnosis of MM is 70 years. We compared the outcomes of upfront AHCT across age groups for newly diagnosed MM in the era of novel therapies using the Center for International Blood and Marrow Transplant Research (CIBMTR) database.
Methods: We analyzed the outcomes of 15,999 MM patients aged 20 years and older from the USA who received a single AHCT with melphalan conditioning within 12 months of diagnosis between 2013 and 2017 and reported to the CIBMTR. Multivariate analysis was performed for non-relapse mortality (NRM), relapse/progression (REL), progression-free survival (PFS) and overall survival (OS) using Cox proportional hazards model with age at transplant in decades as the main effect. Hazard ratio (HR) with 95% confidence intervals (CI) are reported. Because of the large sample size, a p-value of <0.01 was considered significant a priori.
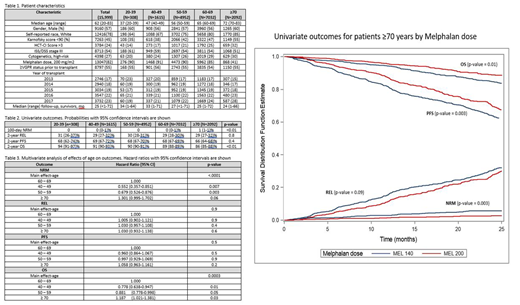
Results: Table 1 shows patient, disease and treatment characteristics by age group at diagnosis. All age groups had similar distribution of gender, race, ethnicity, Karnofsky performance score (KPS), comorbidity index (HCT-CI), stage III by Durie-Salmon/International Staging System. There was a higher proportion of high-risk cytogenetics in patients ≥70 years (30%), compared to age group 40-49 years (24%) and 20-39 years (20%) in this population. Older patients were more likely to be White compared to younger patients: 85% ≥70 compared to 64% 20-39 years. While 82% of the overall population received melphalan 200 mg/m2, 58% of the ≥70% received Mel 140 mg/m2. There were more ASCT performed in the ≥70-year age group in 2017 (28%) compared to 2013 (15%). Univariate outcomes by age groups shown in Table 2 revealed that 100-day NRM was higher in ≥70 years at 1% compared to younger patients (p <0.01) and 2-year OS was lower in ≥70 years at 86 (85-88)% compared to 60-69 years, 89 (88-89)% (p<0.01). When adjusted for other variables (Table 3), compared to reference age group of 60-69 years, patients ≥70 had similar NRM (HR 1.3, 95% CI 1, 1.7, p 0.06), REL (HR 1.03, 95% CI 0.9, 1.1, p 0.6), PFS (HR 1.06, 95% CI 1, 1.2, p 0.2), and OS (HR 1.2, 95% CI 1, 1.4, p 0.02). The leading cause of death across all age groups was primary disease. Among the ≥70 years cohort, melphalan dose was a surrogate for worse outcomes including NRM at 100 days (Mel 140, 1 (1-2)% vs Mel 200 0 (0-1)%, p 0.003, PFS at 2 years Mel 140 64 (60-67)% vs Mel 200 69 (66-73)%, p 0.003, and OS at 2 years (Mel 140 85 (82-87)% vs Mel 200 89 (86-91)%, p 0.01) (Figure).
Conclusions: This is the largest study of AHCT in older adults with MM. More MM patients ≥70 years are being transplanted in the US over time. While our data may highlight referral and access biases regarding which older patients may be referred for ASCT, our results confirm that patients ≥70 years can undergo transplant safely and achieve similar benefits as 60-69 years' old patients. Our results also suggest that melphalan 200 mg/m2 may be given safely in the ≥70 years population. While melphalan conditioning dose 140 mg/m2 in the ≥70 group is associated with worse outcomes, this is likely a surrogate for higher frailty and comorbidities in this cohort of patients. Our analysis confirms that AHCT has similar benefits in terms of disease control (REL and PFS) in both young and older MM patients. This benefit is seen even in a contemporaneous era where proteasome inhibitors and/or immunomodulator drugs are used in upfront treatment. Thus, AHCT remains a safe consolidation therapy across all age groups of MM patients.
Hari:Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Research Funding; Janssen: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Amgen: Research Funding; Spectrum: Consultancy, Research Funding; Sanofi: Honoraria, Research Funding; Cell Vault: Equity Ownership; AbbVie: Consultancy, Honoraria. Kumar:Janssen: Consultancy, Research Funding; Takeda: Research Funding; Celgene: Consultancy, Research Funding. Shah:Poseida: Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Nkarta: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene, Janssen, Bluebird Bio, Sutro Biopharma: Research Funding; University of California, San Francisco: Employment; Genentech, Seattle Genetics, Oncopeptides, Karoypharm, Surface Oncology, Precision biosciences GSK, Nektar, Amgen, Indapta Therapeutics, Sanofi: Membership on an entity's Board of Directors or advisory committees; Indapta Therapeutics: Equity Ownership; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Teneobio: Consultancy, Membership on an entity's Board of Directors or advisory committees. Qazilbash:Genzyme: Other: Speaker; Bioclinical: Consultancy; Amgen: Consultancy, Other: Advisory Board; Autolus: Consultancy. D'Souza:EDO-Mundapharma, Merck, Prothena, Sanofi, TeneoBio: Research Funding; Prothena: Consultancy; Pfizer, Imbrium, Akcea: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal