Introduction: Up to 20-40% of patients (pts) with diffuse large B-cell lymphoma (DLBCL) and Hodgkin lymphoma (HL) and most treated pts with follicular lymphoma (FL) will have relapsed or refractory (RR) disease. Despite recent therapeutic advances, a minority of pts with transplant-ineligible RR HL or DLBCL, or RR FL will achieve durable remission with currently available treatments (tx). Effective novel therapies for pts with RR HL, DLBCL, or FL remain an unmet need.
Although responses to PD1 blockade have been observed in pts with RR HL, DLBCL, and FL, there is room for improvement. Despite a high overall response rate (ORR) to anti-PD1 monotherapy in RR HL, the complete response (CR) is low and most patients with RR DLBCL or FL will not respond. Histone deacetylase inhibitors (HDACi) have immunomodulatory effects, including enhancing antigen presentation, recruiting T-cells into tumors, and promoting T-cell function. Preclinical models in melanoma and lung cancer demonstrated enhanced anti-tumor activity when HDACi were combined with PD1 blockade. We conducted a phase I study to determine the safety and efficacy of pembrolizumab plus vorinostat in RR DLBCL, FL, and HL.
Methods: Adult pts with RR HL, DLBCL, or FL who had failed ≥ 1 prior line of tx and were transplant-ineligible were enrolled to receive IV pembrolizumab and oral vorinostat in 21-day cycles. Pts were treated in a dose-escalation cohort with 2 dose levels (DL) using a Rolling 6 design and then onto an expansion cohort with tx at the recommended phase 2 dose (RP2D). In DL1, vorinostat was administered at 100mg BID on days 1-5 and 8-12 and in DL2, vorinostat was administered at 200mg BID on days 1-5 and 8-12. Pembrolizumab dose was 200mg every 3 weeks in all DLs. Tx could continue for a maximum of 2 years. The primary endpoint was safety and determination of the RP2D. Responses were assessed using PET-CT (DLBCL, HL, FL) or CT (FL) by investigators according to the 2014 Lugano Classification.
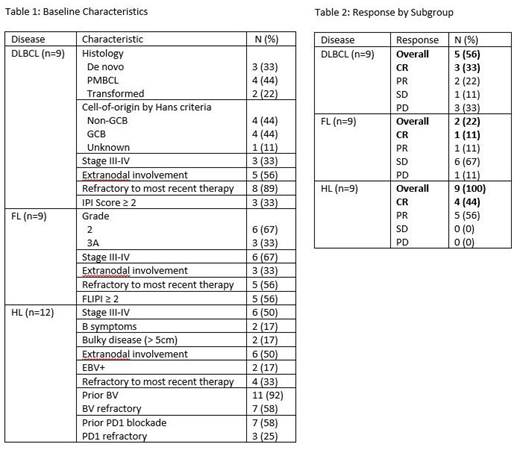
Results: 30 pts were enrolled, including 12 in the dose escalation and 18 in the expansion cohort. At baseline, 67% were male, 73% were Caucasian, the median age was 44 years (range 19-79), the median number of prior tx was 4 (range 1-7), 9 pts had DLBCL, 9 had FL, and 12 had HL. Among DLBCL pts, 4 had primary mediastinal large B-cell lymphoma (PMBL), 4 were non-GCB by Hans criteria, 3 had double-expressor lymphoma, and 3 had prior CAR T-cells. Among HL pts, 11 had prior BV, 7 had prior PD1 blockade, and 3 were refractory to prior PD1 blockade. Additional baseline characteristics are shown in Table 1.
In 28 pts with tx data, the median number of cycles was 5 (range 1-16). Of 6 pts treated at DL1, 1 had a DLT (Grade 4 Stevens-Johnson syndrome, SJS) and 1 out of 6 pts had a DLT in DL2 (Grade 3 pulmonary embolism, PE); therefore, DL2 was chosen as the RP2D. In all pts, including the expansion cohort, the most common adverse events (AEs) were nausea (61%), fatigue (57%), hypertension (54%), anemia (50%), leukopenia (50%), hyponatremia (43%), diarrhea (43%), neutropenia (39%), and thrombocytopenia (39%). Grade (gr) 3-4 AEs included 2 pts with gr 3 neutropenia, 1 pt had Gr 4 SJS, and 1 pt each with gr 3 hypertension, anemia, thrombocytopenia, hyperkalemia, lymphopenia, or PE. Immune-related AEs included the Gr 3 SJS and 5 (18%) pts with thyroiditis. 2 patients had vorinostat dose reduction - 1 for neutropenia, 1 for GI toxicity. 12 pts remain on tx; tx was discontinued for toxicity in 3 pts (SJS, PE, elevated creatinine), stem cell transplant in 3 pts, patient preference in 2 pts, and insufficient response in 10 pts.
Among 27 evaluable pts, the ORR was 59% and the CR rate was 30% (Table 2). The 9 pts with DLBCL had an ORR of 56% with a CR of 33%, including 2 CR, 1 PR, 1 PD in the 4 PMBL pts (1 had been refractory to CAR T-cells). The 9 pts with FL had an ORR of 22% and CR rate of 11%, and the 9 pts with HL had an ORR of 100% with a CR rate of 44% - both evaluable HL pts who were previously refractory to PD1 blockade responded (2 PR). The median follow-up time in all pts was 4 months (mo, range 1-11). The median duration of response, progression-free survival (PFS), and overall survival (OS) in all patients were 6 mo, 8 mo, and not reached. The overall 6 mo PFS and OS were 59% and 76%, including 67%/71% in DLBCL, 33%/40% in FL, and 80%/100% in HL.
Conclusions: Pembrolizumab and vorinostat was tolerable and produced objective responses in pts with RR DLBCL, FL, and HL. A majority of DLBCL pts and all HL pts responded, including pts who had progressed on prior anti-PD1 tx.
Herrera:AstraZeneca: Research Funding; Kite Pharma: Consultancy, Research Funding; Adaptive Biotechnologies: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Gilead Sciences: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Pharmacyclics: Research Funding; Immune Design: Research Funding; Merck: Consultancy, Research Funding; Genentech, Inc.: Consultancy, Research Funding. Popplewell:City of Hope: Employment. Budde:F. Hoffmann-La Roche Ltd: Consultancy. Mei:Seattle Genetics, Inc.: Research Funding. Chen:Autolus Therapeutics: Employment. Kwak:Pepromene Bio: Consultancy, Equity Ownership, Research Funding; InnoLifes: Consultancy, Equity Ownership; Xeme BioPharma, Inc: Consultancy, Equity Ownership; Enzychem LifeSciences: Consultancy; Celltrion Healthcare: Consultancy; Celltrion, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal