Background: The phase II AIM trial (Tam et al, NEJM 2018; NCT02471391), using combination BTK inhibitor ibrutinib (IB) and BCL-2 inhibitor venetoclax (VEN) therapy achieved excellent 16 week complete remission rates (CRR) (primary endpoint; 62%) in patients (pts) with poor prognosis mantle cell lymphoma (MCL), higher than currently approved second line IB monotherapy. Additionally, overall response rate (ORR) by PET of 71%, with attainment of minimal residual disease (MRD) negativity was reported. At the time of primary endpoint analysis (median follow up 15.9 months), median progression free survival (PFS), duration of response (DOR), time to progression (TTP) and overall survival (OS) had not been reached. Herein, we update our results with an additional 21.6 months of follow-up and report the outcomes of pts who electively interrupted treatment.
Methods: Pts with MCL who had relapsed or refractory disease (R/R; n=23) or were treatment naïve but inappropriate for chemotherapy (n=1) were enrolled. Pts commenced treatment with IB 560mg/d for 4 weeks, followed by weekly ramp-up of VEN to 400mg/d. Initially, pts were intended to continue IB and VEN until progressive disease or unacceptable toxicity; a later amendment allowed pts to electively interrupt both drugs if MRD-negative CR was reached. Pts who elected to interrupt therapy were closely monitored by peripheral blood flow MRD testing and regular CT scans, and were allowed to recommence both study drugs at MRD recrudescence or clinical progression.
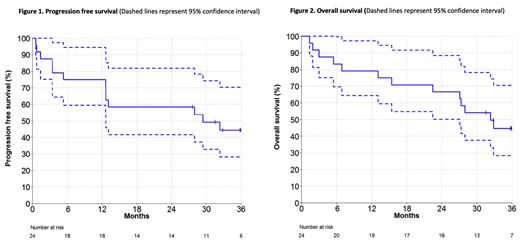
Results: Median age was 68 years (range, 47-81), median number of previous treatments was 2 (range, 0 to 6), and 50% had aberrations of TP53. As previously reported, the flow MRD-negative and ASO-PCR MRD-negative rates were 67% and 38%, respectively. For the current analysis, the median follow-up was 37.5 months (range, 1.4-45.3).The median DOR and TTP had not been reached and were estimated to be 74% and 60% at 30 months, respectively. The median PFS was 29 months (95% CI of 13-NE) (Figure 1), and the median OS was 32 months (95% CI of 27-NE) (Figure 2).
For pts with TP53 aberrant MCL (n=12), the CRR was 50% (95% CI 21-79) with and without PET. In this group the ORR was 58% (95% CI 28-85) without PET and 50% (CI 21-79%) with PET. The DOR in 6 responders with TP53 aberrant MCL (excluding one pt with CRu) was 12+, 24+, 26+, 35+, 36+ and 38+ months from study commencement. In contrast, pts with ATM aberrant MCL (n=10) the CRR was 90% (95% CI 55-100) with and without PET, and the ORR was 90% (95% CI 55-100) with and without PET. Of the 9 responders with ATM aberrant MCL, the DOR was 9+, 9+, 12+, 24+, 31+, 35+, 36+, 37+ and 38+ months from study commencement.
Of 13 deaths, 8 were due to PD. Of the other 5 deaths, 2 were due to infection, and 1 each to cardiac failure, glioblastoma, and graft versus host disease after an allograft that occurred after PD on trial.
Five pts in MRD negative PET-confirmed CR electively interrupted treatment after a median 18.5 months (range 18-33) of therapy: one pt had radiological progression after 7 months, and the other 4 remained free of clinical or MRD progression after 6, 13, 17 and 18 months off therapy.
Conclusions: The median PFS for the IB-VEN combination was 29 months. Treatment interruption was feasible for pts in MRD-negative complete remissions, raising the prospect of limited duration targeted-agent therapy in the management of R/R MCL.
Handunnetti:Gilead: Honoraria; Abbvie: Other: Travel Grant. Anderson:Walter and Eliza Hall Institute: Employment, Patents & Royalties: Institute receives royalties for venetoclax, and I receive a fraction of these.. Hicks:Clarity: Research Funding; Ipsen: Research Funding; Telix Pharmaceuticals: Equity Ownership. Bressel:Regeneron Pharmaceuticals: Consultancy. Koldej:NanoString Technologies: Other: Travel grant. Ritchie:Amgen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy; BMS: Research Funding; Takeda: Research Funding; Beigene: Research Funding; Imago: Research Funding; Novartis: Honoraria; Sanofi: Honoraria. Seymour:Roche: Consultancy, Research Funding, Speakers Bureau; Acerta: Consultancy; Takeda: Consultancy; Janssen: Consultancy, Research Funding; Celgene: Consultancy, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau. Roberts:Walter and Eliza Hall Institute: Patents & Royalties: Institute receives royalties for venetoclax, and I receive a fraction of these.; AbbVie: Other: Unremunerated speaker for AbbVie, Research Funding; Janssen: Research Funding; Australasian Leukaemia and Lymphoma Group: Membership on an entity's Board of Directors or advisory committees; BeiGene: Research Funding. Tam:AbbVie: Honoraria, Research Funding; Roche: Honoraria; BeiGene: Honoraria; Novartis: Honoraria; Pharmacyclics LLC, an AbbVie company: Honoraria; Janssen: Honoraria, Research Funding.
While ibrutinib is FDA approved for use in relapsed and refractory mantle cell lymphoma, venetoclax and combination venetoclax and ibrutinib are not licensed for use in mantle cell lymphoma. The use of venetoclax and ibrutinib for this indication is investigational.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal