Background
Although most patients (pts) with refractory or relapsed B acute lymphoblastic leukemia (r/r B-ALL) achieved complete remission (CR) after CD19 or CD22 CAR T-cell therapy, many of them still relapsed during extended observation period. To predict and prevent the occurrence of relapse, it is critical to understand the clinical and biological factors that affect long-term outcome after CAR therapy. Here, with next generation sequencing (NGS) technology, we monitored 339 somatic gene mutations in 90 pts at enrollment and relapse. We tried to determine whether and how the genetic mutation profile will affect the remission duration in patients who received CD19 or (and) CD22 CAR T-cell therapy.
Methods
During November, 2017 and June, 2019, 90 pediatric r/r B-ALL pts were enrolled in this study including 39 who received CD19 CAR T-cell therapy (Clinical trial #ChiCTR-OIC-17013623), 20 who received CD22 CAR T-cell therapy (Clinical trial #ChiCTR-OIC-17013523), and 31 who received sequential CD19 and CD22 CAR T-cell therapies (Clinical trials #ChiCTR-OIB-17013670 and ChiCTR-ONC-17013648). At enrollment, detailed WHO classification has been evaluated. NGS for 339 somatic gene mutations related to hematological neoplasms has been detected by Illumina sequencing at enrollment and relapse. The average sequencing depth was more than 3000×. Sensitivity of NGS was 0.01% variant allele frequency (VAF). Somatic gene mutations whose frequency in all pts is over 3 times have been involved for analysis. We also analyzed the association of complex chromosome aberrations, high fusion genes with remission duration in these patients
Results
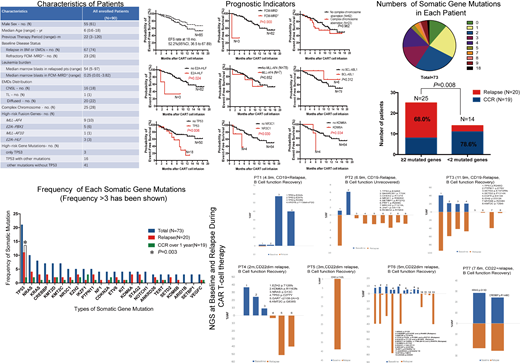
85/90 pts had achieved complete remission after CAR T-cell therapy. 18-month EFS rate of all 85 pts was 52.2% (95%CI, 36.5 to 67.9) including 43 pts who were bridged to transplantation. 21/85 pts had relapsed with a median relapse time of 6 (1-12) months. 6/85 pts died from other reasons. 19/85 pts remained sustained remission defined as remaining remission for at least one year, including 11 pts who had been bridged to transplantation after CAR T-cell infusion. 83 somatic gene mutations were identified across 73 pts (median 2 mutations/patient; range, 0-18). 24 types of somatic gene mutations had been identified over 3 times including TP53, NRAS, KRAS, CREBBP, KMT2D, KMT2C, NR3C1, EZH2, IKZF1, PTPN11, NF1, CDKN2A, ETV6, KIT, KDM6A, STAG2, NOTCH1, ANKRD26, TERT, SETD2, KDM6B, ARID1B, SETBP1and VEGFC. We compared the gene mutation between relapsed pts and pts who had sustained remission over 1 year and found the pts who had more than 2 mutated genes have higher risk of relapse (P=0.008). We also foundTP53 mutation was poor indicator sustainable remission (P=0.003). TP53 gene mutation was detectable as a major gene at relapse with AVFs ranging from 0.55% to 95.03%. Many new derived mutated genes were easily observed at relapse, which might be generated under the pressure of CAR T-cells, and contributed to relapse. We found that FCM-MRD+and E2A-HLF fusion gene were poor indicators of sustained remission (P=0.000 and 0.024). In addition, most relapsed pts had B cell function recovery, and was significantly different to pts who remained sustained remission without bridging to transplant (P=0.001), which indicates the loss of the surveillance of CAR T-cells.
Conclusion
Most r/r B-ALL pts have more than 1 somatic gene mutation before CAR T-cell infusion. Complex gene mutation profile (>2) indicated higher risk of relapse. TP53 mutation was a poor indicator of sustained remission and remained a major genetic feature in blast at relapse. Many new types of gene mutations were detected at relapse. Therefore, monitoring genetic mutations across the procedure of CAR T-Cell therapy may predict the incidence relapse and suggest proper interventions to prevent relapse.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal