Background: BMT is now an integral part of consolidation and/or salvage for patients with AML. With the growing population survivors, there is a need to understand the quality of survival. This would allow for appropriate resource allocation and implementation of risk-based screening for early detection of chronic health conditions over time. Yet, a comprehensive evaluation of the long-term burden of morbidity borne by AML patients treated with BMT remains unknown. We addressed this gap by evaluating long-term severe/life-threatening/fatal chronic health conditions (CHCs) in AML patients treated with BMT using the BMTSS.
Methods: Patients were eligible if they had undergone an allogeneic or autologous BMT for AML between 1974 and 2014 at one of 3 BMT centers in the US, had survived for ≥2y after BMT, and were ≥21y of age at BMT. Of the 1,113 eligible subjects, 711 (64%) participated. BMT survivors identified a nearest-age sibling to constitute an unaffected comparison group (N=1,136). Survivors and siblings completed a 231-item BMTSS survey that included questions regarding CHC diagnosis by their healthcare provider, including age at onset of CHC. Scoring was based on Common Terminology Criteria for Adverse Events ([CTCAE] v 5.0) to determine the severity of CHCs. Using multivariable logistic regression, we determined the risk of any severe (CTCAE grade 3) or life-threatening (CTCAE grade 4) CHC in survivors compared with siblings, adjusting for age at study, sex, race/ethnicity, education, annual household income and insurance status. Information regarding therapeutic exposures (pre-BMT and transplant-related) was abstracted from medical records. Cumulative incidence of CHCs (including fatal [CTCAE grade 5] CHCs) were calculated for BMT survivors, treating relapse-related death as a competing risk.
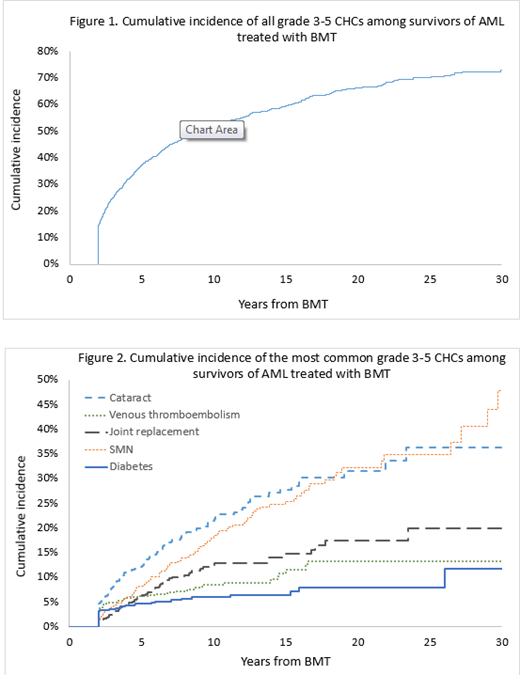
Results: Mean age at BMT was 48.6±13.8y and at survey was 58.2±11.5y. Mean interval between BMT and study participation was 9.7±6.8y; 53% were females, and 78% were non-Hispanic white; 86% received allogeneic BMT (48% from an unrelated donor). Conditioning was Fludarabine/Melphalan-based in 53% and TBI-based in 35%; stem cell source was peripheral blood (70.3%), bone marrow (19.3%), and cord (10.4%). For the siblings, the mean age at survey was 56.9+13.4y; 61% were females, and 88% were non-Hispanic white. BMT survivors vs. sibs: 53.3% of the BMT survivors and 30.4% of the sibs reported grades 3-4 CHCs, placing the survivors at a 3.0-fold higher odds of grades 3-4 CHC (95%CI, 2.4-3.7, p<0.0001). The odds of developing the following CHCs were significantly higher in BMT survivors when compared with siblings: subsequent malignant neoplasms (SMNs: odds ratio [OR]=10.0, 95% CI, 5.5-17.9, p<0.0001), diabetes (OR=5.3, 95% CI, 3.0-9.3, p<0.0001), venous thromboembolism (OR=3.8, 95%CI, 2.5-5.8 p<0.0001), cataracts (OR=3.7; 95%CI, 2.7-5.0, p<0.0001), and major joint replacement (OR=1.5, 95% CI, 1.1-2.1, p=0.02). Among BMT survivors: The 10- and 20y cumulative incidence of a grade 3-5 CHC was 52.0%±1.4% and 66.2%±1.6%, respectively (Figure 1). 75 survivors had developed SMNs: skin (melanoma/ squamous cell [56%]), breast (13%), colon (7%), prostate (7%), and other cancers (24%). The 10- and 20y-cumulative incidence of the most common CHCs were as follows (Figure 2): SMN (10y: 17.9%±1.7%, 20y: 32.1%±2.9%), cataract (10y: 21.5%±1.9%, 20y: 31.5%±3.0%), major joint replacement (10y: 12.5%±0.9%, 20y: 17.5%±3.4%), venous thromboembolism (10y: 8.6%±1.1%, 20y: 13.2%±2.1%), and diabetes (10y: 6.0%±1.9%, 20y: 8.0%±1.5%).
Conclusion: The burden of severe/life-threatening CHCs is substantially higher in BMT survivors when compared with an unaffected comparison group. The incidence of severe/ life-threatening/ fatal chronic health condition following BMT for AML exceeds 50% at 10y, and continues to increase with time, approaching 70% at 20y post-BMT. Subsequent malignant neoplasms, diabetes, thrombo-embolic events, cataracts, and major joint replacement constitute the largest burden of morbidity. These findings suggest the need for increased awareness of the long-term burden of morbidity, to ensure close monitoring of these survivors to anticipate and manage morbidity.
Weisdorf:Incyte: Research Funding; Fate Therapeutics: Consultancy; Pharmacyclics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal