Introduction: Patients (pts) with myelofibrosis (MF) experience a broad array of symptoms that negatively affect HRQoL. Fedratinib (FEDR) is an oral, selective inhibitor of JAK2 that was investigated in the randomized, placebo (PBO)-controlled, phase III JAKARTA study in adult pts with intermediate- or high-risk primary or secondary MF and no prior ruxolitinib exposure. Here we report the effect of FEDR 400 mg (starting dose) vs. PBO on pt-reported MF-associated symptoms and HRQoL in the JAKARTA study.
Methods: Pt-reported symptoms were assessed daily during the week before day 1 of each cycle and at the end of cycle 6 (EOC6) using the modified Myelofibrosis Symptom Assessment Form (MFSAF), which comprises 6 key MF symptoms (night sweats, early satiety, pruritus, pain under ribs on the left side, abdominal discomfort, bone/muscle pain), each scored from 0 (absent) to 10 (worst imaginable). Total symptom score (TSS), ranging from 0-60, was estimated by averaging daily TSS (sum of the individual symptom scores) during the week of each scheduled assessment. HRQoL was assessed at cycle 1 day 1 (C1D1) and EOC6 using the EuroQoL-5D 3-level (EQ-5D-3L), a generic, self-administered questionnaire with 5 dimensions (mobility, self-care, pain, usual activities, anxiety/depression). Health utility scores were derived using UK population preferences, with a range from -0.594 to 1.0. A higher EQ-5D-3L utility score represents a better health state.
All pts randomized to FEDR 400 mg/day or PBO with an evaluable assessment at BL (defined as available daily TSS on the MFSAF for ≥5 of 7 days in the week before C1D1; and non-missing values on the EQ-5D-3L at C1D1) were included in analyses. Cross-sectional assessment of changes from BL at post-BL visits was performed. Differences in mean changes from BL at post-BL visits between Tx groups were compared using a pooled 2-sample, 2-sided t-test. Effect sizes (95% confidence intervals [CI]) for between-group differences in mean changes from BL were also estimated using Hedges' g. Effect sizes between 0.2 (small) and 0.5 (medium) are a commonly used ballpark threshold for clinically meaningful differences between Tx groups. Responder analyses were performed using logistic regression, controlling for BL scores. On the MFSAF, symptom response was defined as a ≥50% reduction in TSS from BL. Pts with a TSS of 0 at BL were excluded from responder analyses. For EQ-5D-3L, a ≥0.08-point change from BL in health utility was used to define clinically meaningful improvement at EOC6. Pts with missing values at post-BL visits were considered non-responders in the responder analyses.
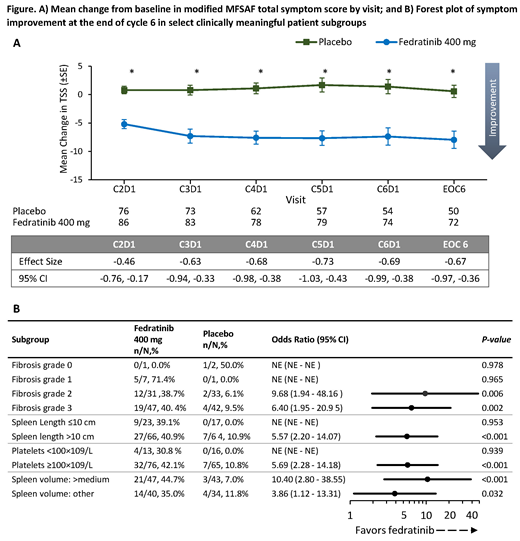
Results: The MFSAF evaluable population comprised 91/96 FEDR 400 mg pts (95%) and 85/96 PBO pts (89%). BL characteristics were comparable between Tx arms. At BL, mean TSS was 17.6 and 14.7 for FEDR and PBO, respectively. Clinically meaningful and statistically significant between-group differences in favor of FEDR were generally observed for all individual MFSAF symptoms and across all visits (above medium effect size: night sweats, abdominal discomfort, early satiety, pain under ribs on left side; above small effect size: bone/muscle pain, pruritis). Mean TSS change significantly favored FEDR vs. PBO across all assessments, with clinically meaningful medium effect sizes (Figure A). Symptom response rate at EOC6 was significantly greater with FEDR (40.4%) than with PBO (8.6%)(odds ratio [OR] 6.97 [95%CI 2.87, 16.9]; P<0.001). Treatment benefit in favor of FEDR was similar across prespecified clinically relevant pt subgroups (see Figure B for selected key subgroups).
EQ-5D-3L health utility scores at BL were similar between Tx arms (0.70 and 0.72 with FEDR and PBO, respectively). The difference in mean change from BL at EOC6 in EQ-5D-3L health utility was also clinically meaningful in favor of FEDR, with an effect size of 0.37 (95%CI 0.08, 0.66). The proportion of pts with clinically meaningful improvement in EQ-5D-3L health utility at EOC6 was significantly higher with FEDR, at 23.2% vs. 6.5% in the PBO arm (OR 5.12 [95%CI 1.81, 14.48]; P=0.002).
Conclusions: FEDR 400 mg provided clinically meaningful and statistically significant improvement in TSS across the 6 MF-relevant symptoms, and broader HRQoL, as measured by health utility, vs. PBO in MF pts with no prior exposure to ruxolitinib. HRQoL benefits of FEDR were observed across clinically relevant pt subgroups, suggesting minimal heterogeneity of Tx effect.
Mesa:Shire: Honoraria; Promedior: Research Funding; Sierra Oncology: Consultancy; Celgene Corporation: Research Funding; Incyte: Other: travel, accommodations, expenses, Research Funding; PharmaEssentia: Research Funding; Gilead Sciences: Research Funding; Genentech: Consultancy; NS Pharma: Research Funding; Galena Biopharma: Consultancy; AOP Orphan Pharmaceuticals: Honoraria, Other: travel, accommodations, expenses; AbbVie: Research Funding; Baxalta: Consultancy; Genotech: Research Funding; LaJolla: Consultancy; Novartis: Consultancy, Honoraria, Other: travel, accommodations, expenses; Pfizer: Research Funding; CTI: Research Funding; Samus: Research Funding. Schaap:Novartis: Consultancy; Celgene: Consultancy. Vannucchi:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ITALFARMACO: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; CTI: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees. Kiladjian:AOP Orphan: Honoraria, Research Funding; Celgene: Consultancy; Novartis: Honoraria, Research Funding. Passamonti:Janssen: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene Corporation: Consultancy, Speakers Bureau. Zweegman:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding. Talpaz:Constellation: Research Funding; Asana: Research Funding; Promedior: Research Funding; Gilead: Research Funding; Celgene: Consultancy, Other: Travel; NS Pharma: Research Funding; Stemline: Research Funding; Novartis: Research Funding; Incyte: Research Funding; Janssen: Research Funding; CTI: Research Funding; BMS: Consultancy; Samus: Research Funding. Verstovsek:Celgene: Consultancy, Research Funding; Gilead: Research Funding; Promedior: Research Funding; CTI BioPharma Corp: Research Funding; Genetech: Research Funding; Blueprint Medicines Corp: Research Funding; Novartis: Consultancy, Research Funding; Sierra Oncology: Research Funding; Pharma Essentia: Research Funding; Astrazeneca: Research Funding; Ital Pharma: Research Funding; Protaganist Therapeutics: Research Funding; Constellation: Consultancy; Pragmatist: Consultancy; Incyte: Research Funding; Roche: Research Funding; NS Pharma: Research Funding. Rose:Celgene Corporation: Employment, Equity Ownership. Tang:Celgene Corporation: Employment, Equity Ownership. Hu:Celgene: Employment, Equity Ownership. Brownstein:Celgene: Employment, Equity Ownership. Guo:Evidera: Employment. Ye:Evidera: Employment. Harrison:AOP: Honoraria; Novartis: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal