After allogeneic hematopoietic cell transplantation (HCT), a relatively small number of donor hematopoietic cells must reconstitute the entire recipient hematopoietic system, while the donor is left with a near normal pool of hematopoietic cells. We hypothesized that the increased replicative demand on donor cells in the recipient after allogeneic HCT will accelerate telomere shortening and magnify the genetic alterations that are associated with normal aging, including clonal hematopoiesis. We aimed to compare mutation frequency in genes associated with clonal hematopoiesis of indeterminant potential (CHIP) and myeloid diseases between donors and recipients, with a focus on transplant pairs with older donors.
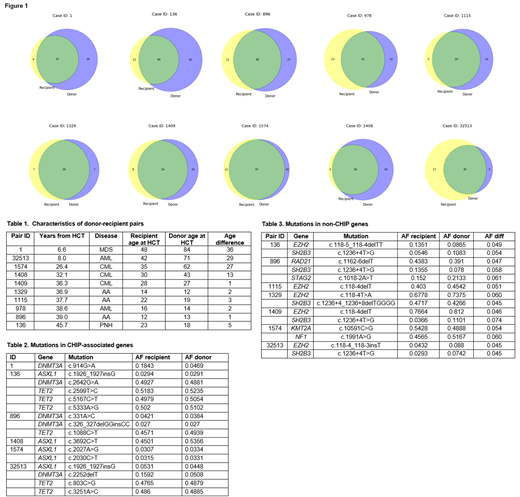
We obtained contemporary blood samples from 10 related donor-recipient pairs surviving a median of 36.6 years (range 6.6-45.7 years) after HCT. Information on donor-recipient pairs is summarized in Table 1. Variant libraries were prepared from bulk peripheral blood mononuclear cells (PBMCs) using Archer Dx VariantPlex Myeloid panel of 75 myeloid disease-associated genes (ArcherDx, Boulder, CO). Sequencing was completed on the Illumina HiSeq system.
Variants with allelic frequency (AF) ≥2% were detected in donors (median number of variants 50.5, range 35-107) and recipients (median number of variants 50, range 32-109). In all pairs, there was significant overlap in the variants detected, although some were unique to donors or recipients (Figure 1). Two of the 3 donor-recipient pairs with >25 years difference in donor age (84 and 71 years) and recipient age (47 and 42 years) showed an increase in the shared variant AF in the recipient in DNMT3A (nonsense and frameshift mutations) of 5 to 18% and 5 to 16%, respectively. All other shared variants in CHIP-associated genes (DNMT3A, ASXL1, TET2) detected in 6 pairs did not show significant difference in AF (Table 2). Among other shared variants of non-CHIP genes, 7 pairs showed mutations with ≥5% difference in AF, but the difference was small (mean difference 5.3%, range 4.5-7.4%) (Table 3).
In conclusion, our results suggest that even decades after transplantation and high replicative demand, most donor variants, at the level of detection of this assay, do not preferentially expand in the recipient. Donor-recipient pairs with older donors and ~30-year difference in age with the recipient showed a modest expansion in DNMT3A clones. Future studies will compare contemporary samples to historical samples from the time of transplant.
Radich:Novartis: Other: RNA Sequencing; TwinStrand Biosciences: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal