Background: Myelodysplastic syndromes (MDS) represent a heterogeneous group of hematopoietic stem cell disorders with risk of progression to AML. Isocitrate dehydrogenase 1 mutations (IDH1m) occur in approximately 3-4% of MDS patients (pts). Olutasidenib is a highly potent, selective small molecule inhibitor of IDH1m that has shown clinical activity in AML (De Botton, 2019).
Methods: The ongoing Phase 1/2 study (NCT02719574) has evaluated the safety, PK/PD, and clinical activity of olutasidenib alone or in combination with azacitidine (AZA) or cytarabine in IDH1m AML/MDS pts. Safety for all pts and efficacy for evaluable pts with MDS in Phase 1 and 2 are reported here. IDH1m variant allele frequency (VAF) was measured at baseline and on treatment by central droplet digital PCR (ddPCR) assay (peripheral blood). Duration of FT-2102 exposure was calculated using Kaplan-Meier methodology; patients on ongoing treatment were censored at the data cutoff date.
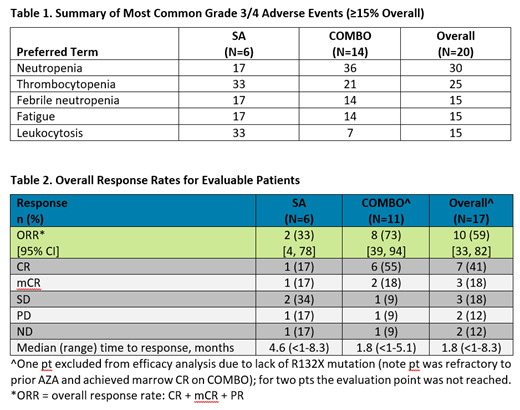
Results: As of the 19-Jun-2019 data cutoff date, 20 pts with IDH1m MDS had received olutasidenib continuously either as a single agent (SA; n=6) or in combination (COMBO; n=14) with AZA (75 mg/m2 × 7 days q 4 weeks). The study population (3 intermediate risk, 12 high risk, 4 very high risk MDS and 1 missing at data cutoff) included 7 newly diagnosed pts and 13 relapsed/refractory pts who had received a median of 1 prior regimen (maximum 4). Eleven pts received prior hypomethylating agents for a median 7.8 months and 2 pts were missing prior treatment records. The median time on treatment for all MDS pts treated with olutasidenib +/- AZA was 8 months (range: <1, 21 months); 6 months (range: 2, 21 months) for SA and not reached for COMBO. Ten (50%) pts remain on treatment (SA, n=2; COMBO, n=8); 10 (50%) pts (SA, n=4; COMBO, n=6) discontinued treatment due to disease progression (n=3), transplant (n=3), other reasons (n=2 [alternative treatment]), adverse event (n=1), or death (n=1). Olutasidenib has been well tolerated both as SA and in combination with AZA. Overall in SA and COMBO, regardless of causality, most treatment-emergent AEs (TEAEs) were grade (Gr) 1/2, with most common (≥20%) TEAEs: nausea (60%); fatigue (50%); arthralgia, constipation, thrombocytopenia (40% each); neutropenia or dyspnea (35% each); vomiting (30%), extremity pain, headache, hematuria, cough or diarrhea (25% each); and myalgia, decreased appetite, hypokalemia, fever, contusion, dizziness, hypoesthesia, pruritis, angina bullosa hemorrhagica or insomnia (20% each). The most common (≥15%) Gr 3/4 TEAEs for the overall population (Table 1) were neutropenia (30%); thrombocytopenia (25%); febrile neutropenia, fatigue and leukocytosis (15% each). One COMBO pt had Gr 3 differentiation syndrome and one COMBO pt experienced Gr 1 QTc prolongation that resolved without treatment interruption. Clinical responses occurred in 33% SA and 73% COMBO (CR 17% and 55%, respectively; Table 2). Updated mutation analyses and other response endpoints will be presented.
Conclusions: Olutasidenib has shown favorable safety profile and clinical activity in IDH1m MDS, with an overall response rate (ORR) rate of 59% (95% CI: 33-82%) and durable disease control. Phase 2 is ongoing at 150 mg BID single agent and in combination with AZA.
Cortes:Immunogen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; BiolineRx: Consultancy; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Sun Pharma: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Biopath Holdings: Consultancy, Honoraria. Wang:Pfizer: Other: Advisory role, Speakers Bureau; Stemline: Other: Advisory role, Speakers Bureau; Daiichi: Other: Advisory role; Amgen: Other: Advisory role; Agios: Other: Advisory role; Abbvie: Other: Advisory role; Kite: Other: Advisory role; Jazz: Other: Advisory role; Astellas: Other: Advisory role, Speakers Bureau; celyad: Other: Advisory role. Watts:Takeda: Research Funding; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Lee:Ai Therapeutics: Research Funding; Karyopharm Therapeutics: Consultancy; AstraZeneca Pharmaceuticals: Consultancy; Roche Molecular Systems: Consultancy; Helsinn: Consultancy; Jazz Pharmaceuticals, Inc: Consultancy. Baer:Abbvie: Research Funding; Astellas: Research Funding; Incyte: Research Funding; Kite: Research Funding; Al Therapeutics: Research Funding; Forma: Research Funding; Takeda: Research Funding. Dao:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding. Dinner:Agios: Consultancy; Pfizer: Consultancy; AstraZeneca: Consultancy. Yang:Agios: Consultancy; AstraZeneca: Research Funding. Recher:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Macrogenics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sunesis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Kelly:FORMA Therapeutics: Employment. Sweeney:FORMA Therapeutics: Employment. Brevard:FORMA Therapeutics: Employment. Henrick:FORMA Therapeutics: Employment. Forsyth:FORMA Therapeutics: Employment. Guichard:FORMA Therapeutics: Employment. Mohamed:FORMA Therapeutics: Employment. Wei:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: AHW is a former employee of the Walter and Eliza Hall Institute and receives a fraction of its royalty stream related to venetoclax, Research Funding, Speakers Bureau; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Macrogenics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Honoraria, Research Funding; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal