Introduction
The acquisition of ABL1 Kinase Domain (KD) mutations represent the most frequent resistance mechanism in CP-CML patients (pts) treated with tyrosine kinase inhibitors (TKI). Currently, the standard assay relies on a poorly sensitive technique, Sanger Sequencing (SS). Thus, the detection of these mutations using SS might be too late to trigger a timely treatment change. In a national phase III academic trial (PETALs, EudraCT 2013-004974-82), we evaluated prospectively the value of a more sensitive technique, Next Generation Sequencing (NGS) to detect KD ABL1 mutations in newly diagnosed CP-CML patients randomized to get nilotinib 600 mg/d for 6 years ± Pegylated-IFN-α2a (Peg-IFN) 45 μg/wk for 2 years in combination.
Methods
Newly diagnosed CP CML pts ≤65 years were randomized 1:1 to get NIL 300 mg BID alone (M0 to M48, arm A) vs Peg-IFN alone for 30 days (M-1→M0) 30 mg/wk as priming, prior to NIL 300 mg BID + Peg-IFN 30 μg/wk 2 weeks, upgraded to 45 μg/wk thereafter, for up to 2 y (M0 to M24, arm B) followed by NIL alone for 4 more years unless pts enter a treatment-free remission phase. In addition to KD mutational analysis performed by SS as per protocol, patients also had KD mutational analysis performed by NGS at M3, M6, M12 and 6-monthly thereafter until achievement of a stable MMR, regardless of response. NGS assay was performed as previously described (Kizilors et al. Lancet Haematol 2019).
Results
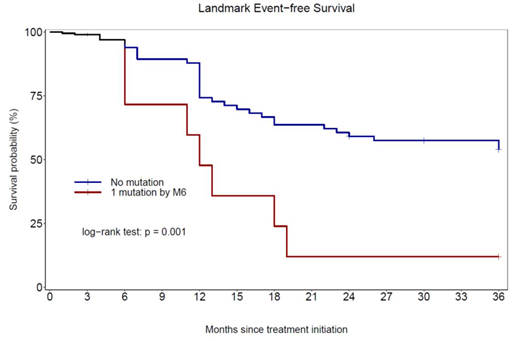
Two hundred pts were randomized (99 in A, 101 in B), of which 96 patients (51/99 in A, 45/101 in B, p=0.399) underwent a KD mutational analysis performed by NGS as part of this study. The remaining 104 patients are currently being screened and the full dataset will be presented. Among the 96 patients tested, there was no difference in the distribution between the 2 arms with respect to gender, age [median 45 years (18-66)] or risk factors distribution (p=0.862 and 0.328 for Sokal and ELTS respectively in patients tested at 3 months). The median follow-up of this cohort is 45.0 (33.2-58.7) months. By 12 months, 11 patients [8/51 (11.8%) in A, 3/45 (6.6%) in B] had developed a KD mutation. After only 3 months of TKI therapy, 3 patients were found mutated (Y253H 2 pts, T315I 1 pt), of whom 2 pts were only detected using NGS. At M6, a KD mutation was found in 8 pts [A: 7 patients, B: 1 pt, (p= 0.055), of which 6/8 were not detected by SS, due to either low level Variant Allele frequency (VAF, n=5) or low level BCR-ABL transcript levels (n=1). Y253H mutations were found in 4 pts, T315I in 2 pts and E255K in 1 pt. Consecutively to KD mutation identification, 6/8 patients lost their response and were withdrawn from study (1 pt with a Y253H detected at M3 progressed to advanced phase), while 1 pt lost MMR at last follow-up and another pt with a mutation sensitive to nilotinib achieved MMR. KD mutations were detected while pts were in optimal response at M6 [BCR-ABL <1% (IS)] in 5 pts (of whom 2 pts were in MMR) and in 1 pt in the warning category [BCR-ABL at 2.2% (IS) with a low level T315I at 2% VAF which became detectable by SS only at M18]. The remaining 2 pts had KD mutations detected at M3 and were in warning and failure respectively at M6 according to ELN 2013. A landmark analysis performed at 6 months showed that patients with KD mutations detected by NGS had a significantly worse event-free survival (EFS, according to ELN, Guilhot J. et al. Blood 2012) compared to pts without KD mutation [25% (8%-83%) vs 64.4% (51.9-80.1%) at 24 months, p=0.001 figure]. Although there were higher proportions of pts with high risk SOKAL and ELTS in the group with mutation, it did not reach statistical significance (p= 0.054 and 0.082 respectively).
Conclusions
This is the first prospective trial to demonstrate that NGS can detect low level KD mutations in CP CML patients treated with first line 2GTKI±Peg-IFN after only 3 to 6 months on therapy before these become detectable by SS and despite achieving an optimal response in BCR-ABL transcript level reduction (according to ELN 2013). The proportion of patients who develop KD mutations by 12 months on upfront 2GTKI should not be underestimated, as their outcome is poor. NGS may trigger early clinical intervention and prevent progression in this group, although a prospective trial is needed in this regard. Finally, the proportion of KD incidence in pts who receive Peg-IFN in addition to nilotinib might be lower compared to those treated with nilotinib alone. Final updated results will be presented.
de Lavallade:Novartis: Honoraria, Speakers Bureau; Incyte biosciences: Honoraria, Research Funding, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; BMS: Honoraria, Research Funding, Speakers Bureau. Jackson:Incyte biosciences: Honoraria, Research Funding, Speakers Bureau. Kizilors:Incyte biosciences: Research Funding. Etienne:BMS: Honoraria, Speakers Bureau; Incyte Biosciences: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau. Huguet:Jazz Pharmaceuticals: Honoraria; Servier: Honoraria; Amgen: Honoraria. Guerci-Bresler:Pfizer: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Incyte biosciences: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau. Rea:BMS: Honoraria; Incyte Biosciences: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees. Mahon:Novartis: Consultancy, Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Incyte Biosciences: Honoraria, Speakers Bureau. Dulucq:BMS: Honoraria, Speakers Bureau; Incyte Biosciences: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau. Nicolini:Incyte Biosciences: Honoraria, Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau; Sun Pharma Ltd: Consultancy.
Pegylated Interferon alpha 2 a is not licensed in this indication
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal