
Background: Cancer-associated thrombosis (CAT) is a leading cause of death in cancer patients after cancer itself. Risk factors for CAT include tumor type/stage, body mass index (BMI), blood cell counts and chemotherapy exposure. These factors form the basis of prediction algorithms for CAT risk, including most notably the Khorana Risk Score. However, significant limitations exist with these currently-available risk prediction models. Emerging data suggest that a tumor's molecular profile can impact venous thromboembolism (VTE) risk. Mutations of ALK, EGFR, IDH1, ROS1, and KRAS for example have been shown to modulate the risk of CAT; however, these studies were limited by the number of mutations and specific tumor types analyzed. We hypothesized that extended molecular testing in a large patient cohort would allow for improved detection of molecular signatures associated with CAT. We analyzed deep-coverage targeted sequencing data (up to 341 genes) of tumor samples from 11,776 cancer patients to identify gene mutations associated with VTE.
Methods: Adult patients with any solid tumor diagnosis who had their tumors sequenced using MSK-IMPACT from 1/2014 to 12/2016 were retrospectively assessed for CAT events using redundant algorithmic methods and individual chart reviews. The endpoint was defined as the first instance of cancer-associated pulmonary embolism and/or proximal/distal lower extremity deep vein thrombosis (DVT). An episode of upper extremity DVT was considered a competing event. The observation period was limited to 365 days after IMPACT blood control sampling. Cause-specific Cox proportional hazards regression was used to test for an association between gene and CAT risk, adjusting for clinical covariates including age, cancer type, cytotoxic chemotherapy (time-dependent), anticoagulant use, stage (metastatic/non-metastatic) and prior history of VTE. Separate multivariate models evaluated the association for the 60 most frequently-mutated genes identified, along with ALK, MET, ROS1 which were included based on existing literature suggesting an effect on VTE risk. Final p-values were adjusted for false discovery using the Benjamini-Hochberg procedure, and the threshold for statistical significance was set at 0.10. Patients with multiple cancer diagnoses were excluded.
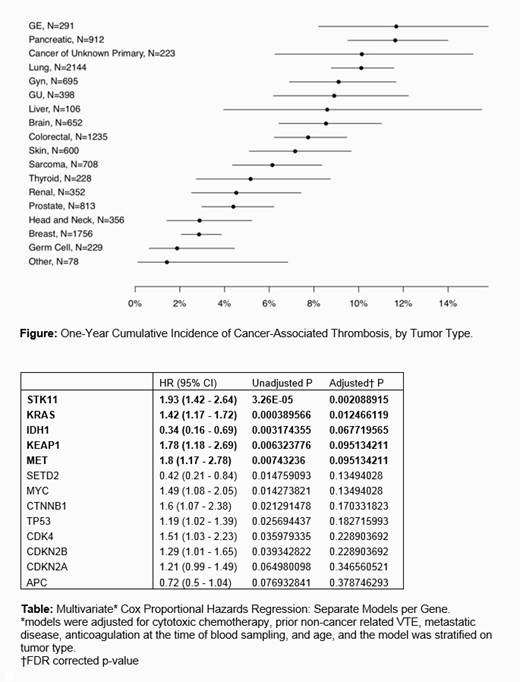
Results: Out of 11,776 individuals we observed 727 CAT events (6.2% of cohort). The most commonly represented tumor types were lung (18%), breast (15%) and colorectal cancer (10%); see Figure for a breakdown of CAT incidence by tumor type. Most (72%) of patients were metastatic at time of IMPACT testing and 4% were on anticoagulation therapy. Statistically significant predictors of CAT included cytotoxic chemotherapy (HR 1.61 [1.37-1.9]; p<0.001), history of VTE preceding cancer diagnosis (HR 2.48 [1.62-3.79]; p<0.001), and presence of metastatic disease (HR 2.48 [1.94-3.16]; p<0.001). Molecular profiling of tumors revealed STK11 (HR 1.93 [1.42-2.64]; adjusted p=0.002), KRAS (HR 1.42 [1.17-1.72]; adjusted p=0.012), KEAP1 (HR 1.78 [1.18-2.69]; adjusted p=0.095), and MET (HR 1.8 [1.17-2.78]; adjusted p=0.095) somatic mutations were associated with a significant increase in the risk of CAT, independent of tumor type. However, it should be noted that a majority of these genes were specific to lung cancer highlighting the prevalence of this tumor type within the IMPACT cohort. Additionally, the presence of IDH1 mutations in primary brain tumors correlated with a decreased risk of CAT in comparison to wild-type IDH1 (HR 0.34 [0.16-0.69]; adjusted p=0.068), consistent with previous studies. See Table for an extended report of regression coefficients.
Conclusions: This work is the first large-scale analysis to elucidate cancer-specific genomic determinants of CAT. Using a large patient cohort, we found that somatic tumor mutations in STK11, KRAS, IDH1, KEAP1, and MET modulate the risk of venous thromboembolism in solid tumor patients. Further analysis is needed to validate these findings and identify additional molecular signatures unique to individual tumor types. We hope these findings will ultimately translate into improved risk stratification for patients at risk of CAT.
Mones:Janssen: Research Funding. Iyengar:Puma Biotechnology: Consultancy; Novartis: Consultancy. Hyman:AstraZeneca: Consultancy, Research Funding; Fount: Consultancy, Equity Ownership; Pfizer: Consultancy; Chugai Pharma: Consultancy; Loxo Oncology: Research Funding; Boehringer Ingelheim: Consultancy; Bayer Pharmaceuticals: Consultancy, Research Funding; Genentech / Roche: Consultancy; PUMA Biotechnology: Research Funding; CytomX Therapeutics: Consultancy. Park:Merck: Research Funding; Parker Institute for Cancer Immunotherapy: Research Funding; Astellas: Research Funding; Ipsen: Consultancy. Khorana:Bayer: Consultancy; Janssen: Consultancy; Sanofi: Consultancy; Pfizer: Consultancy. Soff:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Dova: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees. Mantha:Medical Case Management Group: Consultancy; MJH Live Events: Other: Give CME talk; Heidell, Pittoni, Murphy & Bach, LLP: Consultancy; Daboia Consulting LLC: Equity Ownership; Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal