Lentivector mediated gene transfer into hematopoietic stem/progenitor cells and T cells has resulted in long-term stable integration of transgenes and significant clinical benefits in many diseases. We previously reported (De Ravin et al Sci Transl Med. 2016) early outcome data for 5 patients with X-linked severe combined immunodeficiency (X-SCID) enrolled in a first-in-human lentivector gene therapy clinical trial (NCT03315078) as salvage therapy for older children and young adults who had received prior haplo-identical hematopoietic stem cell transplantation (HSCT) as infants without chemotherapy-based conditioning. Lymphocyte-depleted haplo-identical stem cell transplant in X-SCID infants without conditioning generally results in only a T-cell engraftment that may also decline over time which, combined with B- and NK-cell dysfunction, may result in the progression of multi-system clinical problems (bronchiectasis, infections, gastrointestinal malabsorption, failure to grow, immune dysregulation). By 2016 three additional patients were treated and the cohort of 8 patients have now been followed for 3 to 7 years (Cohort A), where we observed gradual clinical benefit in the clearance of chronic norovirus and associated improved abdominal complaints, malabsorption, and growth and IgG production. Four patients were able to cease immunoglobulin replacement therapy.
Despite these encouraging results, the relatively inefficient transduction of hematopoietic stem/progenitor cells (HSPCs) required large quantities of vector, and resulted in relatively low vector copy number in myeloid cells in some patients, with delayed immune cell recovery and persistent clinical disease especially in the last patient treated, P8. To address this, we developed a refined enhanced transduction (ET) procedure consisting of a single overnight transduction after 48hours pre-stimulation in cytokines (Stem cell factor, Thrombopoietin, Flt3-ligand; 100ng/mL) and incorporated transduction enhancers LentiBoost 1:100 and dimethyl prostaglandin 2 (dmPGE2; 1mM) and recently treat 6 patients, including re-treatment of P8 (Cohort B).
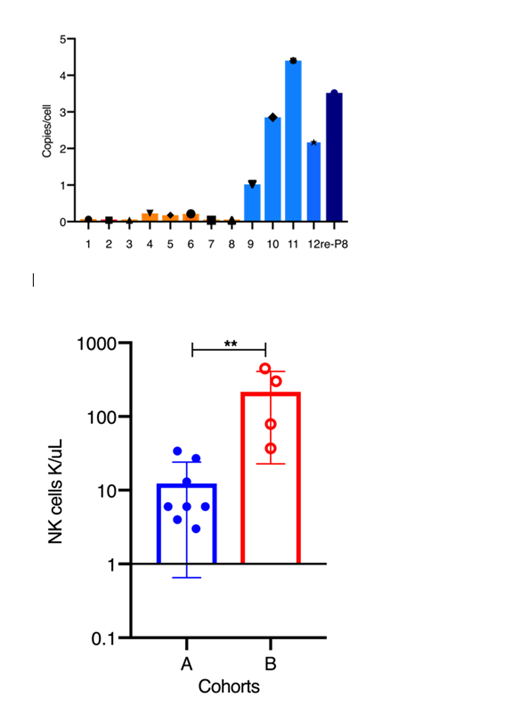
Here we compared the early outcomes from Cohort B patients who received autologous CD34+ HSPCs processed with the enhanced transduction (ET) procedure. These patients (aged 12 to 36 yo) had significant problems with donor T cell infiltration of liver, bone marrow and kidneys, and near absent B and NK cells. Cryopreserved G-CSF/plerixafor mobilized peripheral blood CD34+ HSPCs (5x106/kg to 28x106/kg) were transduced by the ET process at 5% vector concentration, compared to previous method without transduction enhancers of two daily transductions at 20-30% final vector concentration, representing a 10-12 fold reduction in vector used. Colony forming unit assays showed 78%-92% versus 17%-58% vector-positive clones in Cohort B compared with Cohort A, with VCN of 1.5-12 copies (Cohort B), compared with 0.06 to 0.5 copies in Cohort A. At one month following infusion of gene corrected cell product, we isolated peripheral blood CD14+ myeloid cells as indication of early marrow output from engrafted HSPCs, and observed VCN of 1 to 4 copies (P9-12 and re-treat P8), a ≥10x increase from Cohort A (0.04 to 0.23) at same early time point after infusion (Fig 1). In addition to earlier clinical improvement (abdominal complaints, appetite, growth), there was also an early appearance of B and NK cells (Fig. 2) at much higher levels in Cohort B than previously observed even at years after treatment in Cohort A.
In conclusion, we observed significantly improved measures of early clinical outcomes from lentivector gene therapy of older children and young adults with X-SCID using enhanced transduction procedure with addition of LentiBoost and dmPGE2, that achieves much greater transduction efficiencies with >10 fold less vector, and results in faster immune reconstitution and more significant clinical benefit by 3 months. The patients are monitored closely for potential risks from higher VCNs that may be balanced by reduced selection pressure due to the greater numbers of gene corrected clones.
Puck:NIAID: Research Funding; Pfeizer: Other: spouse serves on Rare Disease Advisory Board; Invitae: Other: spouse employment. Cowan:bluebird bio: Consultancy; Homology Medicine: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; UpToDate: Honoraria; California Institute Of Regenerative Medicine: Research Funding; NIH NIAD: Research Funding; Rocket Pharma: Consultancy; Leadiant: Consultancy. Mamcarz:ASSISI Foundation of Memphis: Research Funding; MBIO: Other: St. Jude Children's Research Hospital has an existing exclusive license and ongoing partnership with Mustang Bio for the further clinical development and commercialization of this XSCID gene therapy; California Institute of Regenerative Medicine: Research Funding; NHLBI: Research Funding; UpToDate: Honoraria; American Lebanese Syrian Associated Charities: Research Funding. Gottschalk:Patents and patent applications in the fields of T-cell & Gene therapy for cancer: Patents & Royalties; California Institute for Regenerative Medicine: Research Funding; NHLBI: Research Funding; America Lebanese Syrian Associated Charities: Research Funding; TESSA Therapeutics: Other: Research Collaboration; ViraCyte: Consultancy; MBIO: Other: St. Jude Children's Research Hospital has an existing exclusive license and ongoing partnership with Mustang Bio for the further clinical development and commercialization of this XSCID gene therapy; Sanofi: Honoraria; Tidal: Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy; EMD Serono: Honoraria; ASSISI fundation of Memphis: Research Funding; Inmatics: Membership on an entity's Board of Directors or advisory committees. Meagher:MBIO: Other: St. Jude Children's Research Hospital has an existing exclusive license and ongoing partnership with Mustang Bio for the further clinical development and commercialization of this XSCID gene therapy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal