Background. Treatment failure after allogeneic haematopoietic stem-cell transplantation (AHST) using reduced-intensity conditioning (RIC) results from too much alloreactivity and harmful acute Graft-versus-Host Disease (aGVHD). Studies have identified many reconstituting immune cell subsets associated with development of clinical alloreactivity but the functionally dominant parameters at different time-points remain unknown. We therefore used mass cytometry (MS) to simultaneously assess multiple alloreactive and immunoregulatory cell populations to identify dominant immune reconstitution signatures associated with subsequent development of aGvHD after AHST.
Methods. Phenotypic markers identifying more than 30 immune cell subsets known to influence alloreactivity were combined in a single MS panel. Peripheral blood from 58 patients with haematological cancers was analysed after T-replete HLA-matched RIC-AHST using uniform conditioning. Normalization of individual test samples spiked with CD45-barcoded healthy control cells was used to reduce batch effects. Complementary high-dimensional analytic tools were used to generate cellular profiles across the whole cohort and identify differences between patients grouped by subsequent development of aGVHD.
Results. Unsupervised clustering analysis identified 40 phenotypically distinct T, B and NK cell clusters post-transplant. Significant batch effects were effectively reduced with a novel R-based algorithm normalising data to control cells. Cluster diversity analysis early post-transplant demonstrated lower cluster diversity in patients who subsequently developed aGvHD consistent with perturbation of phenotypic clusters in these patients. Two specific clusters were significantly different in abundance at D+30 in patients who went on to develop aGvHD and those who remained aGVHD-free. A cluster with a CD56brightCD16negCD27+/- regulatory NK cell (NKreg) phenotype was reduced in patients going on to develop aGvHD using both Phenograph and FlowSOM algorithms (p=0.001). These findings were validated by forward analysis using the CITRUS algorithm, revealing a similar differentiating cell population. CD56bright NKreg reconstitution was independent of CMV reactivation and did not impede reconstitution of WT1 and PR1 tumor-associated antigen-specific T cells. The reduction in NKreg in patients who subsequently developed aGvHD was accompanied by a significant increase in alloreactive CCR5+CD45RA-CCR7- CD4 effector memory T cells (Tem).
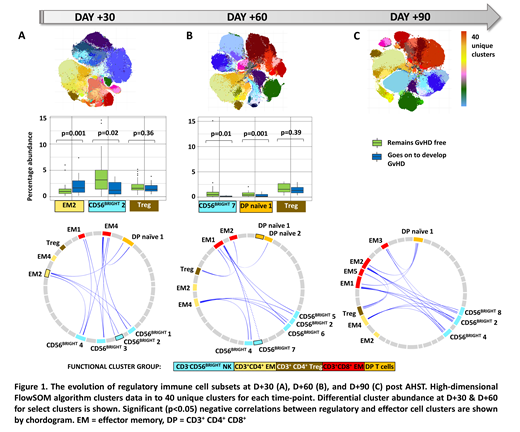
We next used correlation analysis of cluster abundance across the whole cohort to identify all clusters contributing to the immune 'regulome' (those inversely correlated with alloreactive CD4 EM and/or CD8 EM T cell clusters). Notably, at D+30 the regulome consisted of 4 phenotypically distinct CD56bright NKreg clusters and a CD4+CD8+ double positive (DP) cluster, but not FOXP3+ CD4 regulatory T cells (Treg), Figure 1A
Both the identity of differentiating clusters between patients subsequently developing aGVHD and those who remained aGvHD-free, and the dominant constituents of the regulome changed over time. By D+60 a CD56bright NKreg cluster (with a distinct phenotype to the differentiating cluster identified at D+30) and a DP T cell cluster were significantly reduced in patients subsequently developing aGvHD. The D+60 regulome consisted of multiple distinct CD56bright clusters, a DP T cell cluster and CD4 Treg, Figure 1B. Importantly by D+90 the immune regulome consisted of a reduced number of CD56bright NKreg clusters and increasing dominance of CD4Treg, Figure 1C.
Conclusion. We show proof-of-concept that a novel acquisition and analysis pipeline can be applied to MS data to identify multiple immunoregulatory cells after AHST that contribute to the control of reconstituting alloreactive T cells. This approach identified a loss of NK cell-mediated control of alloreactive CD4 Tem cells as the dominant immune process preceding the development of aGvHD early post-transplant. Importantly, we show that specific immunoregulatory subsets are dominant at different time-points, with increasing influence of DP T cells and CD4 Treg at later time points. Our data provide mechanistic insight into the dynamic pattern of control of alloreactivity over time and show that strategies to expand or potentiate immunoregulatory cells to prevent aGvHD should be time-dependent.
Gribben:Acerta/Astra Zeneca: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal