INTRODUCTION
There has been a quantum leap in the research of inhibitor drugs following the discovery of tyrosine kinase inhibitor (TKIs), Imatinib in the early 1990s. The development of Imatinib, the first BCR-ABL tyrosine kinase inhibitor revolutionized the long term outcome of Chronic Myeloid Leukemia (CML) patients. CML is one of the most common hematological malignancies diagnosed in India. The breathtaking success due to effectiveness and financial viability of Imatinib among Indian population even led to its sale in its generic formulations by pharmaceutical companies in India around early 2000s.
METHODS
This is a single-center retrospective observational study conducted among 232 CML patients diagnosed and followed up over 12 years from 2003 until 2015 at Kasturba Medical College and Hospital, Mangalore, Manipal University, Karnataka, India. We closely analyzed the handwritten files and the electronic medical records of each of 232 CML patients for their demographic details, symptoms, date of diagnosis, blood counts, bone marrow reports, spleen size at diagnosis, cytogenetic and molecular reports, date - dosage - change in dose - resistance - side-effects of Imatinib and outcome. Patients were followed up closely.
Results
Median age was 44 years (range, 10 -78 years). In the year of 2008-2012, 46.1% of patients diagnosed with CML. 66.4% were male and 33.6% were female. During the first evaluation of patients at the time of diagnosis, 90.9% of patients were in the chronic phase, 4.7% in accelerated phase and 4.3% in blast phase. 97.8% were started on Imatinib orally as first line. 176 (75.9) patients received 400 mg orally. Five patients chose not to be treated. The most common side effect of Imatinib among patients were as follows: abdominal pain (13.3%), fever (10.8%), general weakness (4.7%), blurred vision (4.7%), abdominal distention (3.4%) and joint pain (2.2%). Response to treatment with Imatinib was evaluated in terms of molecular response as measured by real-time RT Q-PCR.
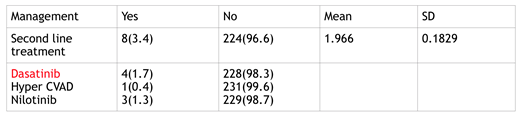
The 3.4% who failed to respond to Imatinib, 3.4% patients were started with second-generation TKI's (dasatinib, nilotinib, hyper CVAD cyclophosphamide, vincristine, Adriamycin and dexamethasone). The first choice for switching TKI therapy in 4 (1.7%) patients were dasatinib, in (3.1%) was nilotinib and only one patient had to use Hyper CVAD chemotherapy. Table has summarized the use of second-generation TKI's. One patient expired and 4 has progression. 181(78.0%) patients came throughout the year of 2015. Among all patients 200 (86.2%) are still alive with disease, 18 (7.8%) are alive without disease and 14 (6.0%) were expired. Overall survival (OAS) is 218 (96%) Progression free survival (86.78%). The median BCR ABL/ABL value at the end of the six months was 11% and at the end of the one year was 3.38%.
Conclusion
The introduction of Imatinib mesylate had phenomenal changes in the management of CML. Molecular response evaluation after six months can predict the subsequent molecular response and can also be used as a surrogate monitor of the marrow cytogenetic response to imatinib therapy in CML. We report a high overall (OAS) and progress free survival (PFR) rates with very less side effects.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal