Ponatinib is a third generation tyrosine kinase inhibitor (TKI) indicated in the treatment of CML-chronic phase (CP), accelerated phase (AP) and blast phase (BP) as well as in patients with the gatekeeper T315I mutation. TOPASE is the real life observatory initiated in France with the participation of 40 CML centers. We report here the interim results of the first 46 patients included in the study as of July 2019, which represents one of the largest real-life Ponatinib study to date in CML.
Methods and Aims: CML Patients (Pts) > 18 years old, with any stage of disease treated with Ponatinib for a period of less than 6 months or prospectively, were included in the study since February 2018 (ambispective study). The principal aims were the evaluation of efficacy (haematological, cytogenetic and molecular responses), overall survival as well as the safety profile of the use of Ponatinib. After a period of 2 years of inclusion, 2 years of follow-up will be performed until 2022. The study will include 150 patients.
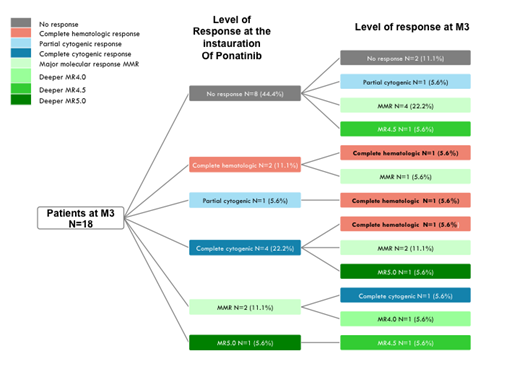
Results: 48% of pts were female and 52% males and the median age was 57 +/-18 years. 87% of pts were in CP, 8.7% in AP and 4.3% in BP. The Sokal score was high (34.8% of Pts) intermediate (19.6% pts ) and low (28.3 % pts), not available in 17.4 %. The initiating dose was 15mg in 19 pts, 30mg in 16 pts and 45 mg in 11 pts. All patients with AP/BP except one, were treated with 45 mg/d. Previous therapies were 2 lines (44.4% of pts), 3 lines (26.7% pts), 1 line (17.8% of pts), 4 lines (6.7% of pts), and 5 lines (2.2% of pts) of TKI. The majority of pts had previously Dasatinib (77%) and Imatinib (70%) whereas 43% received Nilotinib and 31% Bosutinib. The last TKI administered prior to inclusion was Dasatinib (40.9% of pts) , Bosutinib (25% of pts) Nilotinib (18.2% of pts) , Imatinib (11.4% of pts) , Ponatinib (2.3% of pts) and ABL001 (2.3% of pts). There was no significant dose-initiation difference with regard to the previous TKI used. The main reason for the initiation of Ponatinib was failure and poor response to previous therapies (63% of pts) followed by intolerance (28.3% of pts). An ABL kinase mutation was detected in 11 patients during their TKI therapy. In 4 pts, the mutation was a T315I and in these pts the last TKI therapy was Nilotinib (n=2), Dasatinib ( n= 1) and Bosutinib ( n= 1). In 7 other patients, previous mutations detected were mostly in p-loop, with previous therapies including Dasatinib, Nilotinib, Imatinib and Bosutinib. One patient previously treated with Dasatinib, Nilotinib and Ponatinib (last therapy) had a F317L mutation, and two pts treated previously with 2 lines (Imatinib, Dasatinib) and 3 lines (Imatinib, Dasatinib, Nilotinib) had E255V/C1135 and F359C / E450K mutations, respectively. At the time of inclusion, the cardiovascular (CV) history of pts (n=46) included high blood pressure (HBP) (35% of pts) or other CV history (26.9%) including peripheral vascular disorders and ischemic heart disease. In the majority of pts with HBP, the initiating dose of Ponatinib was 15 mg/d ( 41%). Other significant medical disorders included diabetes ( 21.7% of pts ) and dyslipidemia ( 8.7% of pts). Results of the efficacy were evaluated at +3 Months (M3) and at +6 months (M6). M3 analyses were available on 18 pts (Figure 1) and M3+M6 analyses on 14 pts. At the entry of the study, the status of the 18 evaluable pts at M3 was as follows: no CHR (n=8), CHR (n=2), no CCyR ( n= 1) CCyR (n= 4), MMR (n=2), MR5 (n=1). At M3, CHR was obtained in 6/8 pts and 4 out of 6 attaining in the same time MMR and 1/6 reaching MR 4.5. For the remaining 10 pts, MMR was obtained in 5/10, and deep molecular responses in 4/10. At M6, out of 14 pts available for molecular analyses, 6 were in MMR, and 3 were in deep molecular responses. 3 pts had no response to Ponatinib. Analysis of responses in individual pts showed that in 8 pts unresponsive to previous therapies 2 had MMR and 1 MR 4. In 10 pts in CHR or absence of CyR, deep molecular responses were obtained in 5/10 pts. Overall, global improvement of responses was obtained in 55.6% at M3 and 60% in M6. At the time of the interim analysis no significant AE were noted for the 46 pts available for analysis.
Conclusion: Ponatinib in real life situation is a highly efficient therapy in TKI-resistant and intolerant CML pts. Overall improvement was obtained in 60% of pts with early molecular responses, despite 2 or more previous TKI therapies in 70% of pts. The updated molecular results will be presented concerning M3, M6 and M9 timepoints.
Funding : Incyte Biosciences, France
Guerci:INCYTE: Consultancy, Honoraria. Rousselot:Pfizer: Research Funding; Incyte: Research Funding. Huguet:Servier: Honoraria; Jazz Pharmaceuticals: Honoraria; Amgen: Honoraria; Incyte Biosciences: Honoraria; Pfizer: Honoraria; BMS: Honoraria; Novartis: Honoraria. Coiteux:Pfizer: Honoraria; BMS: Honoraria; Novartis: Consultancy, Honoraria; Incyte: Consultancy, Honoraria. Berger:Incyte: Consultancy, Honoraria. Etienne:Novartis: Consultancy, Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Incyte Biosciences: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau. Turhan:novartis: Honoraria, Research Funding; Incyte: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal