Patients with newly diagnosed chronic myeloid leukemia (CML) frequently receive imatinib. Although initial response rates are high, imatinib fails in up to 40% of patients because of disease resistance, frequently because of BCR-ABL kinase domain mutations, or side effects. Patients who discontinue imatinib may have a response to second-generation tyrosine kinase inhibitors (TKIs). Ponatinib (PON) is a potent oral TKI active against unmutated and mutated BCR-ABL kinase. PON is indicated also in Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) patients. Clinical activity of PON was confirmed in the phase II PACE trial, however lack of real world data is evident.
The aim of this non-interventional study was to analyze data on PON treatment and its efficacy in the Czech patients with CML / Ph+ ALL. The study was designed as a one-off retrospective data collection from 3 national registry databases INFINITY, CAMELIA and DATOOL ALL in the period 2014-2018.
In total, the study comprised 27 patients treated with PON at 7 centers; 16 (59.3%) patients were treated for chronic phase (CP) CML, 4 (14.8%) patients for accelerated- or blast-phase (AP/BP) CML and 7 (25.9%) patients for Ph+ ALL. The 16 CP CML patients (68.8% males) had median age at the start of PON treatment 59.7 years (range 28.6-81.4), were heavily pretreated (75% with ≥ 3 TKIs) with median time from diagnosis to start of PON treatment 4.8 (0.2-16.8) years; 37.5% of them had mutations (18.8% with T315l), 75.0% had comorbidities. The 11 AP/BP CML / Ph+ ALL patients (54.5% males) were younger with median age 56.6 (31.2-74.7) years, were less pretreated (36.4% with ≥ 3 TKIs) with a shorter time from diagnosis to start of PON treatment 2.0 (0.4-22.0) years; almost twice as many (72.7%) of them had mutations (54.5% with T315l), 63.6% had comorbidities. The most common reason for switching to ponatinib was hematologic resistance (37.5% of CP CML and 54.5% of AP/BP CML / Ph+ ALL patients). The other most frequent indications were cytogenetic resistance, non-hematologic and hematologic intolerance (18.8%, 12.5% and 12.5% of CP CML patients and 9.1%, 9.1% and 0% of AP/BP CML / Ph+ ALL patients, respectively).
Interestingly, starting dose of PON was 45 mg/day as recommended in the product SmPC only in of about half of the patients (43.8% of CP CML and 54.5% of AP/BP CML / Ph+ ALL patients). Median treatment duration was 16.1 (0.8-49.9) months in CP CML patients and only 2.9 (0.2-36.1) months in AP/BP CML / Ph+ ALL patients. Early (in the first 3 months) and late termination of the treatment occurred in 12.5% and 31.3% of CP CML and 54.5% and 27.3% of AP/BP CML / Ph+ ALL patients, respectively. Disease progression was the major reason for treatment termination (50%). In terms of safety, only 1 patient discontinued therapy due to congestive heart failure, and 1 due to vascular adverse event although more than half of the patients had cardiovascular comorbidities and history of cardiovascular disease.
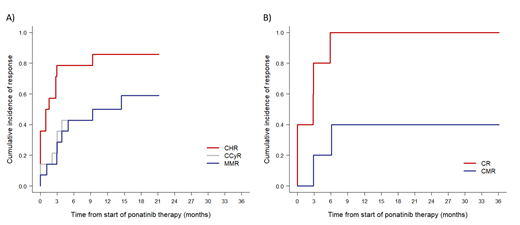
PON efficacy was evaluated in 14 CP CML patients and 5 AP/BP CML / Ph+ ALL who were treated beyond 3 months (Figure 1). More than half of CP CML patients achieved MMR (57.1%) and 40% of AP/BP CML / Ph+ ALL achieved undetectable disease. Estimated percentage of CHR, CCyR and MMR in CP CML patients after one year of treatment was 85.7%, 50% and 50%, respectively. Nevertheless, 5 (35.7%), 2 (14.3%) and 1 (7.1%) patients had CHR, CCyR and MMR at start of PON treatment, respectively. In AP/BP CML / Ph+ ALL patients, estimated percentage of CR and CMR after one year was 100% and 40%, respectively. However, 2 patients (20%) had CR at the start of PON treatment.
Despite limited number of patients, our analysis confirmed PON efficacy in real-life setting with a significant proportion of heavily pre-treated patients achieving durable molecular responses in both CP and AP/BP CML / Ph+ ALL groups. Our data are comparable to the PACE trial results. This study partially fills the gap in RWE data and significantly contributes to the evaluation of real-life clinical practice in rare disease area.
Figure 1. Cumulative incidence of responses on ponatinib treatment in A) CP CML (N=14) and B) AP/BP CML / Ph+ ALL (N=5) patients that continued ponatinib treatment beyond 3 months.
Žáčková:Bristol Myers Squibb: Consultancy; Novartis: Consultancy; Angelini: Consultancy; Incyte: Consultancy. Kellnerová:Angelini Pharma: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal