Study Rationale
The present study with ponatinib is based on previous studies on the potential role of imatinib discontinuation, to achieve a stable treatment-free remission (TFR) of patients with Philadelphia-positive Chronic Myeloid Leukemia (CML). As ponatinib has shown to induce deeper molecular responses compared with imatinib, the rationale is that ponatinib treatment could increase the proportion of patients who could discontinue treatment successfully.
Purpose
The purpose is to determine the successful TFR within the first 48 weeks following cessation of treatment in patients who achieved MR4 on imatinib, and maintained MR4 on ponatinib, after a switch from imatinib.
Eligible patients have been previously treated with imatinib as unique tyrosine kinase inhibitor (TKI) therapy, for at least 4 years, and have documented MR4 (at least 12 months) at the time of ponatinib to study entry.
Objectives
The Primary Objective is to evaluate the proportion of patients without confirmed loss of MR4 or loss of MMR (do not require confirmation).
The Key Secondary Objectives are:
To evaluate the proportion of patients without confirmed loss of MR4 or loss of MMR within 72 and 96 weeks following ponatinib cessation.
To estimate progression-free survival (PFS) from the date of ponatinib cessation to the date of the earliest event.
Treatment-free survival (TFS) defined as a lack of any of the following: loss of MMR, confirmed loss of MR4, re-start of imatinib treatment, progression of AP/BP, or death from any cause.
Overall survival (OS), defined as the time from the date of cessation of ponatinib therapy to the date of death from any cause.
Proportion of patients who regain MR4 within 48 weeks of imatinib treatment re-initiation, following confirmed loss of MR4 within 48 weeks subsequent to ponatinib cessation.
Kinetics of BCR-ABL transcript level (IS) after re-start of imatinib therapy.
Other Secondary Objectives include adverse events, laboratory data for hematology, biochemistry, and urinary test, vital signs and ECGs.
Exploratory Objectives include phenotypic and genotypic biomarkers, as well as functional analysis of cytotoxic cell activation. Plasma monitoring of ponatinib levels will also be performed.
Study Design
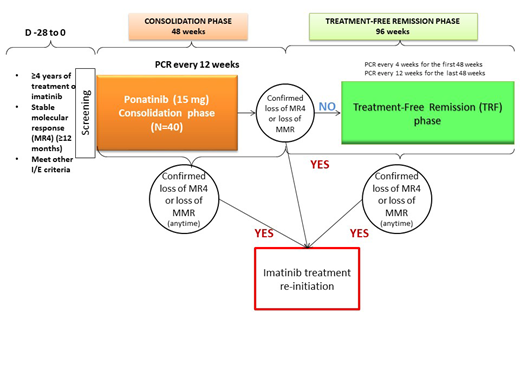
This is a single-arm, open label study, open label study in 40 patients who achieved and maintained MR4, to determine the rate of successful TFR in both gender patients, treated with 15 mg/day of ponatinb for 48 weeks. Ten Spanish sites will participate. The study has two main phases: ponatinib consolidation (48 weeks) and ponatinib TFR phase (96 weeks).
Inclusion criteria are patients who had received a minimum of 4 years imatinib as unique TKI therapy, have documented MR4 at least 12 months prior to study entry, and will continue with MR4 before the discontinuation of ponatinib. After stopping ponatinb (TFR phase), BCR-ABL wil be monitored every 4 weeks during the first 48 weeks, and every 12 weeks during the last period of 48 weeks. Exclusion criteria include patients with transplant, atypical transcripts, CML treatment resistant mutation, or having cardiovascular or pancreatitis diseases. Figure 1:
Current State of the Study
First Visit First Patient: 17 JUL 2019
Patients Enrolled: 4
Patients Recruited: 3
Screening Failure: 0
In Screening: 1 patient
Sanchez-Guijo:Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Amgen: Honoraria; Roche: Honoraria. Hernandez Boluda:Incyte: Other: Travel expenses paid. Steegmann:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal