Introduction: MCL is an aggressive type of non-Hodgkin's lymphoma, and was reported associated with early relapse and poor long-term survival. Treatment options include chemotherapy, immunotherapy, and molecular targeted therapies. As of 2019, molecular targeted therapies available in the United States indicated for the treatment of MCL include the proteasome inhibitor bortezomib, the immunomodulatory drug lenalidomide (following two previous lines of therapy), and the Burton's tyrosine kinase inhibitors (BTKIs) ibrutinib and acalabrutinib (following at least one previous line of therapy).
Objective/Methods: To examine the real-world treatment patterns of patients with MCL globally, a systematic literature review was performed (2010-2019) with predefined methodology and inclusion and exclusion criteria. Embase and Medline were searched via ProQuest and the Cochrane Controlled Register of Trials (CENTRAL) via the Cochrane Library.
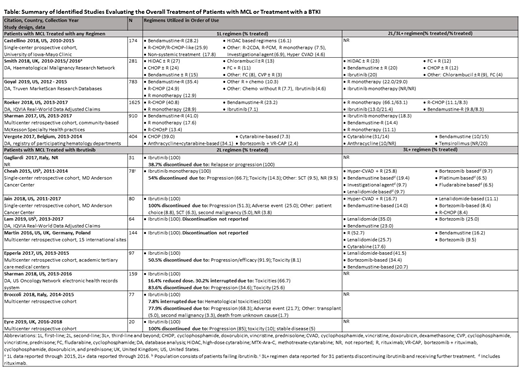
Results: Of the 2207 publications identified, 6 publications (US, n = 4; EU, n = 2) provided information on the first-line treatment of MCL (Table). The most commonly administered first-line treatments were bendamustine-rituximab; high dose cytarabine ± rituximab; and cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone (CHOP) ± rituximab although differences were noted across studies. Most patients received rituximab first-line either in combination with chemotherapy (54.2%-87.5%) or as monotherapy (12.9%-28.9%); although in some studies, rituximab maintenance therapy could not be excluded. The most commonly administered second-line therapies were cytarabine, rituximab monotherapy, and ibrutinib while third-line therapies were rituximab monotherapy, ibrutinib, and temsirolimus.
Nine studies provided data on the real-world treatment of MCL with the BTKI ibrutinib (EU, n = 3; US, n = 5; EU/US, n = 1; Table); no real-world studies were identified for acalabrutinib. Six studies enrolled patients only with relapsed or remitting MCL; 3 studies enrolled patients (≤7.5%) who received ibrutinib as first-line therapy. Ibrutinib second-line therapy was administered to 13%-20% of patients and third-line therapy to 21% of patients. Ibrutinib discontinuation rates in 7 studies varied from 38.7%-83.6%. Non-response, including relapse or progression (34.6%-100%), was the main cause of discontinuation, followed by toxicity/adverse events (8.1%-25.6%). Across studies, toxicity/adverse events causing ibrutinib discontinuation included atrial fibrillation, bleeding/hemorrhage, chronic obstructive pulmonary disease, diarrhea, herpes zoster, infection, leukocytosis/ lymphocytosis, lung cancer, myelodysplastic syndrome, and thrombocytopenia. Two studies provided information on ibrutinib dose reductions (16.4% of patients) and ibrutinib dose interruptions (7.8%-30.2% of patients). Treatment options administered post-ibrutinib included rituximab (52.7%), hyper-CVAD + rituximab (16.7%-25.8%), lenalidomide-based regimens (9.7%-41.5%), and bortezomib-based regimens (8.4%-34.4%).
Conclusion: Our analyses showed that most patients with MCL received first-line chemoimmunotherapy, although regimens varied across studies. Approximately 13%-21% of patients received ibrutinib following first-line therapy. Most ibrutinib discontinuation was due to progression followed by toxicity/adverse events. Upon discontinuation of ibrutinib, considerable variation in treatments was seen and no standard therapy identified. Given the limitations of current therapies, there is a need for additional second- and third-line treatments for patients with MCL. Quantitative assessments of clinical endpoints from real-world studies evaluating BTKI therapies are also warranted.
Yang:BeiGene, Ltd.: Employment. Lesher:Pharmerit: Employment. Lucas:Pharmerit: Employment. Caver:BeiGene, Ltd.: Employment. Tang:BeiGene, Ltd.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal