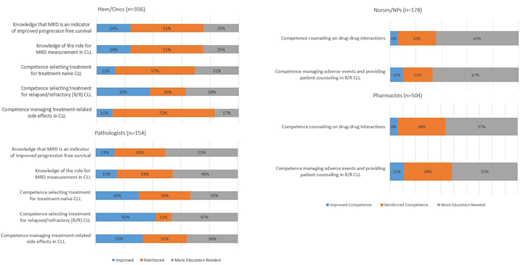
Introduction: Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in the Western world. Treatment selection in CLL is dependent upon a number of factors, such as patient age and genetic mutations, and can be challenging. A number of novel therapies and combination treatment approaches are now available, making treatment selection and management of adverse events increasingly complex. We sought to determine if a curriculum of online continuing professional education (CPE) activities could improve hematologists/oncologists (hem/onc, H/O), pathologists (path), nurse/NP, and pharmacist knowledge, competence, and confidence related to clinical decision making in patients with CLL. Methods: An expert panel identified educational gaps related to the treatment of patients with CLL. Based on the educational needs identified, the curriculum included 4 activities that were posted online between March 2019-June 2019. The first activity was a 30 minute video lecture (1 faculty) about measurable residual disease (MRD) analysis in CLL. The second activity was 30 minute video roundtable discussion (3 faculty) about treatment initiation and selection in newly diagnosed CLL. The third activity was a text activity focused on relapsed/refractory (R/R) CLL with 2 patient cases. The final activity was a video discussion between a nurse and pharmacist about mitigating side effects and optimizing compliance with oral therapies in CLL. Three activities were certified for physicians and the fourth activity was certified for nurses and pharmacists. Multiple-choice questions were asked before and after participation in each activity A repeated-pairs analysis was conducted where individual learners served as their own controls. Improved indicates an incorrect response pre-activity and a correct response post-activity. Reinforced indicates a correct answer pre- and post-activity. Improved confidence indicates a higher level of confidence post-activity. Results: As of July 2019, there were 356 hem/oncs, 154 pathologists, 178 nurses/NPs, and 504 pharmacists included in this analysis. The curriculum had a large impact on the knowledge and competence of hem/oncs and pathologists. MRD is an indicator of improved progression free survival: 13% H/O and path improved their knowledge, 58% H/O and 36% path reinforced their knowledge. The role for MRD measurement in CLL: 24% H/O and 15% of path improved their knowledge, 51% H/O and 39% path reinforced their knowledge. Selecting therapy for treatment-naïve CLL: 13% H/O and 30% path improved their competence, 57% H/O and 36% path reinforced their competence. Selecting therapy for relapsed/refractory (R/R) CLL: 39% H/O and 42% path improved their competence, 26% H/O and 11% path reinforced their competence. Managing treatment-related side effects in CLL: 11% H/O and 33% path improved their competence, 72% H/O and 31% path reinforced their competence. 10% of nurses/NPs and 11% of pharmacists improved and 30% of nurses/NPs and 38% of pharmacists reinforced their skills counseling patients about adverse event management and drug-drug interactions in R/R CLL. Tailoring frontline therapy in treatment-naïve CLL: 26% H/O and 15% path improved their confidence. Tailoring therapy in R/R CLL: 34% H/O and 31% path improved their confidence. Using MRD in CLL management: 31% H/O and 21% path improved their confidence. Improving patient engagement by using effective interprofessional communication: 25% nurses/NPs and 36% pharmacists improved their confidence. Conclusions: This analysis shows that an online CPE curriculum, utilizing many different formats (video, text, panel discussions) can improve and reinforce the knowledge, competence, and confidence of hem/oncs, pathologists, nurses/NPs, and pharmacists in multiple areas surrounding the treatment of patients with CLL. Results also suggest the following areas warrant further education: knowledge of the role for MRD in CLL management, individualizing therapy selection for treatment-naïve and R/R CLL, and managing treatment-related adverse events of CLL therapies. Acknowledgements: Sameer Bhagavatula contributed to data analysis for this research.
Allan:Verastem Oncology, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy; Janssen: Consultancy, Honoraria; AbbVie, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees; Acerta Pharma: Consultancy; Sunesis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics LLC, an AbbVie company: Consultancy; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Awan:Pharmacyclics: Consultancy, Research Funding; AstraZeneca: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Janssen: Consultancy; Genentech: Consultancy; Sunesis: Consultancy; Gilead: Consultancy. Brander:AbbVie: Consultancy, Honoraria, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy; Tolero: Research Funding; DTRM Biopharma: Research Funding; BeiGene: Research Funding; MEI: Research Funding; Acerta: Research Funding; Novartis: Consultancy; Genentech: Consultancy, Honoraria, Research Funding; Teva: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Research Funding. Cohen:Genentech, Inc.: Consultancy, Research Funding; Janssen Pharmaceuticals: Consultancy; LAM Therapeutics: Research Funding; UNUM: Research Funding; Hutchison: Research Funding; Astra Zeneca: Research Funding; Lymphoma Research Foundation: Research Funding; ASH: Research Funding; Takeda Pharmaceuticals North America, Inc.: Research Funding; Gilead/Kite: Consultancy; Seattle Genetics, Inc.: Consultancy, Research Funding; Bristol-Meyers Squibb Company: Research Funding. Barrientos:Pharmacyclics: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Janssen: Consultancy; Gilead: Consultancy; Genentech: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal