Introduction: Clinical trial development and enrollment are pivotal to advancing cancer outcomes. Novel treatment modalities such as Chimeric Antigen Receptor (CAR) T-cell therapy is an intensive therapy that has altered the landscape of hematologic malignancy therapies, with several FDA approvals based on Phase I-II studies. Strict eligibility criteria are implemented to ensure safety of trial participants; however, these criteria can lead to barriers to patient enrollment, hinder the generalizability of the study, and result in a population of participants not representative of those who would benefit from therapy. The aim of this proposal is to characterize inclusion and exclusion criteria in clinical trials for CAR-T cellular therapy in adults with hematologic malignancies.
Methods: The U.S. National Library of Medicine's Clinical Trial database of privately and publicly funded clinical studies was accessed June 2019 to assemble a list of studies with the following filters applied: hematologic, recruiting, not yet recruiting, not recruiting, active, completed, suspended, terminated studies, interventional studies, CAR, CAR T, chimeric antigen receptor, CAR NK, adult, older adult, early phase 1, phase 1, phase 2, phase 3. From this, 95 studies populated, 84 were utilized in this study and 11 studies excluded due to non-hematologic malignancy.
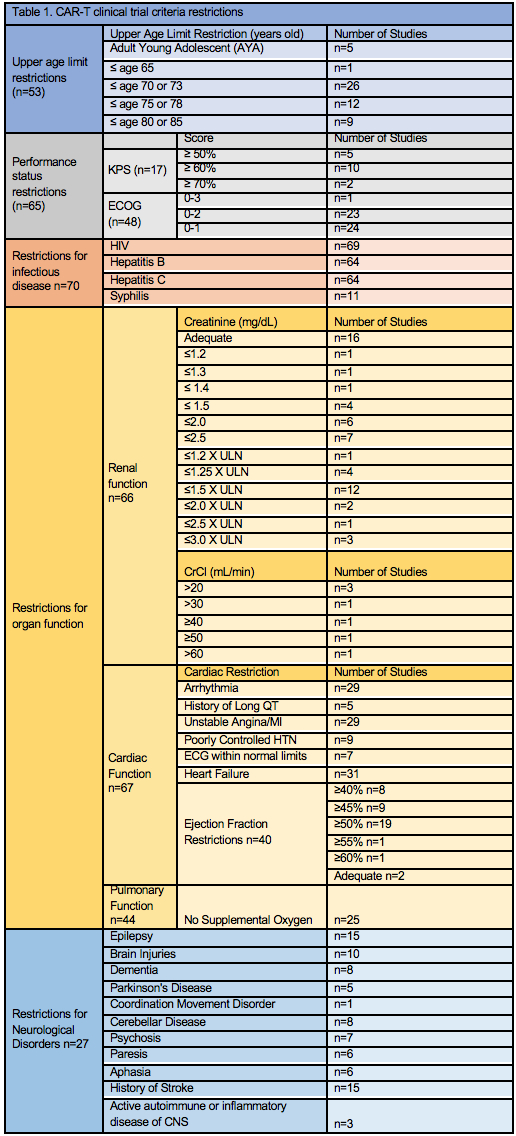
Results: We analyzed 84 CAR-T clinical trials targeting multiple diseases (Table 1) including; acute lymphoblastic (n=7) and myeloid leukemia (n=2); lymphoma (n=6); multiple myeloma (n=40); multiple hematologic malignancies (n=27) and other (n=2). The majority of studies were phase 1 (n=47) or phase 1/2 (n=28). Upper age limit restrictions were in place for 53/84 (63%) of trials. Trials included the AYA population (n=5), ≤ age 65 (n=1), ≤ age 70 or 73 (n=26), ≤ age 75 or 78 (n=12), ≤ age 80 or 85 (n=9). Of the 84 trials, 65 (77%) had performance status inclusion criteria, most commonly was status ECOG 0-2 (n=23) and ECOG 0-1 (n=24). Patients were excluded for a history of a separate or concurrent malignancy in 52/84 (62%) trials, CNS disease was excluded in 45/84 (54%) trials and 70/84 (83%) clinical trials excluded infectious diseases; HIV (n=69) and Hepatitis B/C (n=64). Many studies had restrictions for impairment in organ function; renal impairment (n=66), cardiac deficits (n=67), and abnormal pulmonary function (n=44). Unique to CAR-T trials, 27/84 had restrictions in place for neurological disorders: epilepsy (n=15), history of brain injury (n=10), dementia (n=8), coordination/movement disorder (n=6), cerebellar disease (n=8), psychosis (n=7), paresis (n=6), history of stroke/aphasia (n=21), and active autoimmune or inflammatory disease of the central nervous system (n=3).
Conclusion: CAR-T cellular therapy is a tremendous therapeutic advancement in the medical community. This study emphasizes, in detail, highly variable cross-study inclusion/exclusion criteria for early phase CAR-T studies. This new and promising therapy is actively being studied in a highly select group of patients and may not be generalizable to the older adult with hematologic malignancies due to non-uniform trial criteria. The applicability of this modality should be tempered by the understanding that CAR-T trials have overt age caps, ambiguous performance and comorbidity exclusions, and neurologic exclusions and play a role in limiting patient accessibility to novel clinical trial therapy. Confirmatory prospective and observational studies of CAR-T therapy in representative populations are a high priority.
1. Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. 2016 127:3321-3330. Doi: 10.1182/blood-2016-04-703751
2. Kim ES, Bruinooge SS, Roberts S, et al. Broadening Eligibility Criteria to Make Clinical Trials More Representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J Clin Oncol. 2017;35(33):3737-3744. doi:10.1200/JCO.2017.73.7916
3. Unger JM, Cook E, Tai E, and Bleyer A. The Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. American Society of Clinical Oncology Educational Book. 2016; 36:185-198. Doi:10.1200/EDBK\_156686
Wildes:Janssen: Research Funding; Carevive: Consultancy. Olin:Spectrum: Research Funding; Novartis: Research Funding. Artz:Miltenyi: Research Funding. Jaglowski:Unum Therapeutics Inc.: Research Funding; Kite: Consultancy, Other: advisory board, Research Funding; Juno: Consultancy, Other: advisory board; Novartis: Consultancy, Other: advisory board, Research Funding. William:Guidepoint Global: Consultancy; Defined Health: Consultancy; Techspert: Consultancy; Celgene Corporation: Consultancy; Kyowa Kirin, Inc.: Consultancy. Rosko:Vyxeos: Other: Travel support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal