Background: Although BCP-ALL patients can achieve complete remission (CR) after induction chemotherapy, about 40% of them have residual leukemia cells in the bone marrow which is called minimal residual disease (MRD). Complete MRD response is defined as lack of MRD with an assay sensitivity of >= 10-4, which, if sustained for more than five years, may be regarded as a functional cure for ALL. On the contrary, presence of MRD is a signal of high risk of relapse leading to poor health outcomes. Blinatumomab is the only drug approved for BCP-ALL patients with MRD based on the results from BLAST, a single-arm phase 2 study conducted to assess the efficacy and safety of blinatumomab in adult BCP-ALL patients in CR with MRD. Currently, there is limited evidence on the relationship between MRD response and functional cure in ALL and life years gained.
Objectives: The objective of this study was to assess the relationship between complete MRD response after blinatumomab treatment and 1) functional cure; 2) life years and quality adjusted life years (QALYs) gained among BCP-ALL patients with MRD.
Methods: The relationship between MRD response, and clinical outcomes was analyzed using an existing cost-effectiveness analysis (CEA) based on combined decision tree and Markov cohort model over a lifetime horizon (50 years). The model includes survival conditional on treatment and MRD status for time to stem transplant, relapse and death. In the CEA, blinatumomab was compared to standard of care (SOC, defined as conventional maintenance chemotherapy). Clinical probabilities were derived primarily from blinatumomab clinical trial data and a non-interventional cohort study (used as a historical control). Life years gained were adjusted for quality based on utility values from trial EQ-5D scores. An annual discount rate of 3.5% was applied to estimate future outcomes.
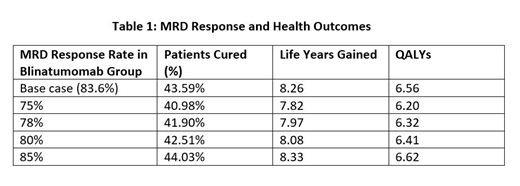
Results: In the base case, the complete MRD response rate was 83.6%, and blinatumomab yielded more life years gained (8.26 vs. 5.68) and more QALYs (6.56 vs. 4.44) compared with SOC. The proportion of patients achieving a functional cure was 43.59% in blinatumomab group vs. 26.78% in the SOC group. Clinical response varied with variations in MRD response rate at 75%, 78%, 80%, 85%. Specifically, when the complete MRD response rate increased from 75% to 85%, the total life years gained in the blinatumomab group increased by 0.51, QALYS increased by 0.42, and the proportion of patients cured increased by 3.05%.
Conclusion: Achieving complete MRD response is associated with longer life expectancy, higher probability of being cured, and more QALYs among patients with BCP-ALL and MRD.
Shao:Amgen: Employment. Tiwana:Amgen: Employment, Equity Ownership. Despiegel:Amgen: Employment, Equity Ownership. Dirnberger:Amgen: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal