Introduction Advances in pediatric Acute Lymphoblastic Leukemia (ALL) have led to substantial improvement in remission rates. However, there is a lack of data characterizing morbidity during induction. During this period, patients are stratified into "standard risk," receiving a 3 drug induction regimen of steroids, vincristine, and asparaginase, or "high risk" receiving anthracycline as a fourth chemotherapeutic agent. An understanding of adverse events (AEs) may facilitate supportive care and predict and guide hospitalization needs during this phase of treatment. There is potential benefit in comparing AE rates between the two treatment groups. This study aimed to identify rates of clinically significant AEs, hospital readmissions, and intensive care unit (ICU) admissions in pediatric patients with ALL during induction.
Methods This retrospective cohort included patients ages 1 through 21 who initiated induction therapy for ALL between January 1, 2013 and May 13, 2018 at Children's Healthcare of Atlanta (CHOA). Algorithms to identify and grade AEs according to Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 definitions were determined a priori. Using these algorithms, manual chart abstraction to identify AEs occurring from diagnosis to day 34 after the start of induction was performed for 19 AEs: pancreatitis, hepatotoxicity, hypoxia, adult respiratory distress syndrome (ARDS), hyperglycemia, thromboembolic event, stroke, infection, sepsis, fever, hypertension, hypotension, seizure, ileus, anaphylaxis, hyponatremia, and constipation. Chart abstraction additionally identified ALL risk classification, admissions to the intensive care unit (ICU), and readmissions. Demographic data were extracted in an automated fashion from the electronic health record. Rates of each AE, readmissions, and ICU admissions were calculated for the induction period. The highest grade of each AE was captured for these analyses and clinically relevant grade 2-5 AEs are reported. Development of each AE, readmission, and ICU admission were stratified by therapy risk group and analyzed by chi square, Fisher's exact, or Kruskal Wallis tests as appropriate.
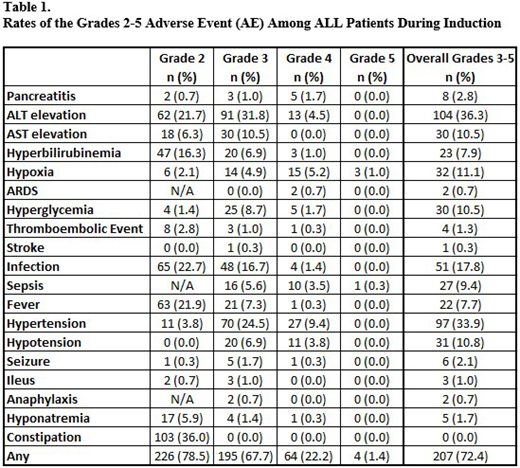
Results AE grading and abstraction are complete and reported on 286 of 512 eligible patients; median age at diagnosis was 5.7 years; 45.1% were female, 47.6% were non-Hispanic white, 20.5% were black, and 23.6% were Hispanic. Among those discharged before the end of induction (283/286), 36.0% were readmitted. Additionally, 12.9% of all patients were admitted to the ICU at some point during induction. Overall, 72.4% of patients had at least one AE > grade 3 during induction. The most common clinically relevant AEs were: ALT elevation (36.3%); hypertension (33.9%); and infection (17.8%) (Table 1). Four of the 286 patients died during induction; 3 deaths were attributed to respiratory failure, and 1 to sepsis. Overall, 36% of patients required readmission with no difference in readmission rates by treatment group. However, patients with high risk treatment were significantly more likely to have an ICU course (18.0% vs 8.2%, p=0.01). A larger proportion of high risk patients experienced sepsis (6.3% vs 3.8%, p=0.05) or hypotension requiring medical intervention (6.6% vs 3.5%, p=0.05).
Conclusions This study demonstrates that AEs ≥ grade 3 are common in induction and that more than a third of patients require readmission during induction after initial discharge. While capture of grade 3-5 AEs indicate severe events, several grade 2 AEs are clinically relevant to morbidity and patient management, such as thromboembolism and fever. These results highlight the AEs driving morbidity during induction therapy, and can be used to guide patients regarding expected AEs. Work is ongoing to analyze the full cohort. Further analyses will compare rates of the highest grade AEs, length of hospitalization, readmission, and ICU admission by chemotherapy regimen.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal