Background
Although serum B12 assay is the most commonly used routine test for assessing cobalamin status in the body, it has its limitations (Mackenzie F and Devalia V (2018) Laboratory performance of serum B12 assay in the United Kingdom (UK) as assessed by the UK National External Quality Assessment Scheme for haematinics: implications for clinical interpretation. BLOOD, 132, suppl 1, 2230).
Holotranscobalamin assay(HoloTC), also known as 'Active B12' assay, is an alternative test which is increasingly used as a first line test since it is felt to represent the assessment of the functional component of the cobalamin status of the body, and possibly a more relevant assessment clinically. However, its technical performance in terms of reliability and suitability in a routine diagnostic laboratory for clinical assessment has not been published.
In the United Kingdom, there are over 30 laboratories performing the assay using five different platforms. The numerical value obtained of any sample can vary considerably between the different methodologies used..
External quality assessment of the assays is organised by UK NEQAS for Haematinics by sending three serum samples every 3 months. An 'all participants' consensus mean is calculated and used as the target value and the results analysed with respect to intra-group variation. The percentage bias from the target value is used to assess performance.
Aim
We present data on one such assessment to demonstrate the performance of the serum holotranscobalamin assay (Survey number 248, April 2018) and also how it is interpreted by the laboratory for clinical use.
Method
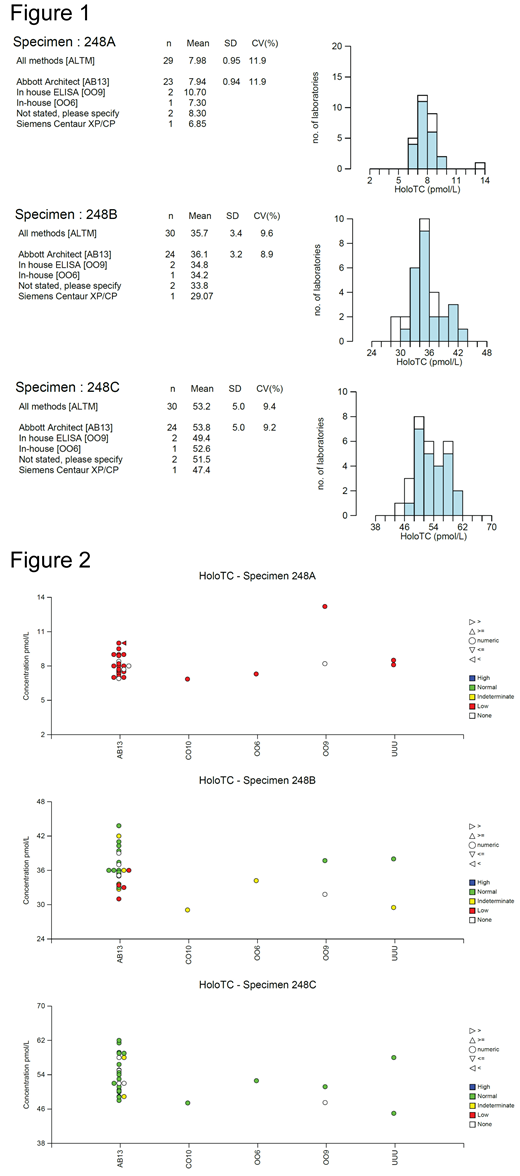
Three serum samples with a HoloTC target value of approximately 7.98 (low/indeterminate), 35.7 (normal/indeterminate) and 53.2 (normal) pmol/L were sent to participating laboratories for analysis (labelled as 248A, B and C respectively: see figure 1). Laboratories were also asked for an interpretation of their result which would be reported to the requesting clinician, namely from low to high (see figure 2).
Results
Fig 1 shows an individual laboratory's result in relation to all laboratories using the same technology (shaded histogram) or all methods (open histogram). There is a significant variation with an overall co-efficient of variation of around 10% within all the three different samples. Fig 2 shows the distribution of results in the different methodologies used and how each laboratory interpreted its result. It demonstrates the bias of results obtained by the different methods.The vast majority use the Abbot Architect (AB13) platform, and there is a suggestion of a trend of the results obtained to lie on the lower side than the other platforms used. For sample 248A, all laboratories reported it as 'low' or deficient. For sample 248B, there is a significant variation is reporting the sample as 'low', 'normal' or 'indeterminate' within the same platform used, clearly seen in the Abbot Architecture group. Indeed, the interpretation provided by the laboratory varies even with the same numerical value of the result.
Discussion
These data demonstrate that serum HoloTC assay has an overall co-efficient of variation around 10%. The numerical value obtained of any sample can vary considerably according to the technology used, and the clinical interpretation provided by the laboratory can be variable and not entirely concordant with the numerical result of the assay used. This is particularly evident around the 30-40 pmol/L range. This may be partly explained by the fact that it is not quite clear what would be regarded as the normal or reference range, which has been previously taken as 40 - 200 pmol/L (according to previous publications).
Conclusion
The UK NEQAS Haematinics Programme is unique in providing external quality assessment for laboratories using HoloTC assays for determining body cobalamin status in a style that is also unique across EQA/ PT schemes. Laboratories need to assess their performance in analysis of serum HoloTC levels in order to provide appropriate clinical advice. Ideally clinicians should be aware of the limitations of the HoloTC assay as demonstrated in this external quality assessment scheme exercise.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal