Introduction: Previous studies have examined the total healthcare resource utilization (HCRU) of patients with beta-thalassemia in relation to the general population. However, limited studies have examined the impact of red blood cell transfusion (RBCT) burden on broad aspects of HCRU beyond transfusion costs among patients with beta-thalassemia.
Methods: Patients with beta-thalassemia in Taiwan's National Health Insurance Research Database (NHIRD) in 2016 were identified (International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] of D56.1). The index date was the first medical claim in the database after 2001. Identified patients were followed from the index date until the end of the study period (December 31, 2016). During the follow-up period, RBCT units and HCRU (all-cause and thalassemia-related) were measured. Thalassemia-related HCRU was defined as any HCRU claim accompanied by a thalassemia or beta-thalassemia diagnosis code. To control for the different lengths of follow-up between patients, both RBCT units and HCRU were reported as the average per 12 weeks over the entire follow-up period. Patients were categorized into 4 cohorts based on the average number of RBCT units received per 12 weeks during follow-up: 0 RBCT units; > 0 to < 6 RBCT units; ≥ 6 to < 12 RBCT units; or ≥ 12 RBCT units. HCRU outcomes of interest were hospital admissions, hospitalized days, outpatient visits, and emergency room (ER) visits. Descriptive statistics were computed to describe HCRU observed in each cohort.
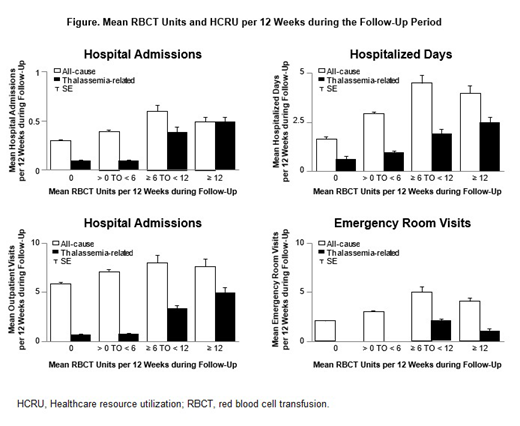
Results: A total of 2,984 patients with beta-thalassemia were included in the analysis, with a mean follow-up of 6.87 years. Mean age at index was 37.8 (standard deviation 23.7) years, and 1,903 (63.8%) patients were female. A total of 1,616 (54.2%) patients did not receive RBCT units during the follow-up period. Of the remaining 1,368 patients, 1,112 (81.3%) received > 0 to < 6 RBCT units, 112 (8.2%) received ≥ 6 to < 12 RBCT units, and 144 (10.5%) received ≥ 12 RBCT units per 12 weeks during follow-up. Mean all-cause and thalassemia-related HCRU was higher for transfused patients than for non-transfused patients across all HCRU categories. Thalassemia-related hospital admissions, hospitalized days, and outpatient days all increased as the transfusion burden increased. Patients in the cohort with the highest average transfusion burden (≥ 12 RBCT units per 12 weeks) had numerically greater mean thalassemia-related hospital admissions (0.5; standard error [SE] = 0.04), hospitalized days (2.5; SE = 0.21), and outpatient visits (4.9; SE = 0.41) than the other cohorts (Figure).
Conclusions: Patients with beta-thalassemia and higher average transfusion burden during the follow-up period had additional HCRU compared with patients who required fewer RBCT units. These data may support physician and payer understanding of the downstream economic impact of RBCT burden in beta-thalassemia.
Tang:GSK: Consultancy; Roche: Research Funding; Pfizer: Research Funding; Janssen: Research Funding; Amgen: Research Funding. Furnback:Sanofi: Consultancy; Regeneron: Consultancy; Celgene Corporation: Consultancy; Abbott: Consultancy; Astellas: Consultancy; Pfizer: Consultancy; Eli Lilly: Consultancy; Janssen: Consultancy; Johnson & Johnson: Consultancy; Gilead: Consultancy; Novocure: Consultancy; Progentec Diagnostics: Consultancy; Becton Dickinson: Consultancy; AstraZeneca: Consultancy; Bristol-Myers Squibb: Consultancy. Wang:Gilead Sciences: Consultancy, Equity Ownership; Celgene Corporation: Consultancy, Equity Ownership; Regeneron Pharmaceuticals: Consultancy, Equity Ownership; Novocure: Consultancy; Pfizer: Consultancy; Eli Lilly: Consultancy; Johnson & Johnson: Consultancy; Astellas: Consultancy; Amgen, Vertex Pharma, Illumina, Biogen, Alexion Pharma, Incyte, Biomarin Pharma, Seattle Genetics, Sarepta Therapeutics, Array Biopharma, Ionis Pharma, Sage Therapeutics, Mylan NV, Neurocrine Biosciences, Bio Techne Corp, Jazz Pharma, Alnylam Pharma, Blue: Equity Ownership. Tang:Asclepius Analytics: Employment. Huang:Celgene Corporation: Employment. Tang:Celgene Corporation: Employment, Equity Ownership. Musallam:Celgene Corporation: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal