Background: Transthyretin amyloidosis cardiomyopathy (ATTR-CM) is a life-threatening, irreversible condition, which can lead to heart failure (HF) and, ultimately, heart transplant or death. Despite the recent approval in United States of a TTR stabilizer (VYNDAQEL®-tafamidis meglumine;VYNDAMAX™-tafamidis) for the treatment of ATTR-CM, disease progression still occurs. This study aims to determine if treatment with AKCEA-TTR-LRx (ION-682884), an antisense oligonucleotide (ASO), is safe and superior to placebo in reducing the risk of cardiovascular (CV) death or CV clinical events in patients with hereditary (hATTR-CM) or wild-type ATTR-CM (wtATTR-CM).
Study Design and Methods: AKCEA-TTR-LRx (ION-682884) is a follow-on compound that incorporates the Ligand-Conjugated Antisense (LICA) technology; in this case, a triantennary N-acetyl galactosamine (GalNAc) moiety which targets the asialoglycoprotein receptors (ASGPR) expressed abundantly on the hepatocyte cell surface. In comparison to inotersen, its parent compound, ION-682884 requires a lower dose and frequency of administration (27-fold smaller; 45mg SC Q4W) to achieve a similar reduction in ATTR, providing greater patient convenience.
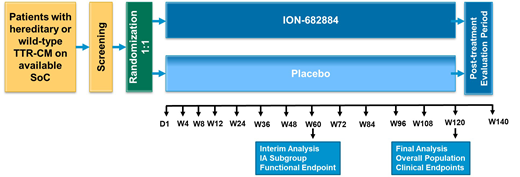
ION-682884-CS2 (EudraCT No: 2019-002835-27) is a Phase 3 global, double-blind, randomized, placebo-controlled study assessing the efficacy and safety of AKCEA-TTR-LRx (ION-682884) in hATTR-CM or wtATTR-CM patients receiving available background standard of care (SoC) therapy. Approximately 750 patients with a history of HF due to ATTR-CM will be randomized 1:1 to receive AKCEA-TTR-LRx (ION-682884) or placebo administered by subcutaneous injection once every 4 weeks. The main inclusion criteria include confirmed diagnosis of ATTR-CM by tissue biopsy or positive PYP/DPD scan, end-diastolic interventricular septum thickness of >12mm, NT-proBNP >600 pg/mL, NYHA class I-III and 6-minute walk distance (6MWD) >150 m. The main exclusion criteria include estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73m2, platelet count below the low limit of normality and urine protein/creatinine ratio (UPCR) ≥ 1000 mg/g. Patients are allowed to concomitantly receive tafamidis/tafamidis meglumine as SoC for ATTR-CM, if locally approved and available, per physician's discretion. The study consists of a 120-week Treatment Period and a 20-week Post-Treatment Evaluation Period. During each study visit, subjects will undergo laboratory tests, cardiac assessments (echocardiography), and functional evaluations. Patient-reported outcomes (PRO) will also be collected. Primary efficacy endpoint is the composite of CV mortality and frequency of CV clinical events (HF-related urgent visits requiring administration of IV diuretics and/or CV-related hospitalizations) at Week 120 study visit, analyzed by the Finkelstein-Shoenfeld method. This test is based on the principle of each patient in the study being compared with every other patient in a pairwise manner in hierarchical fashion. Secondary endpoints include the change from baseline in the 6MWD, Kansas City Cardiomyopathy Questionnaire score, rate of CV mortality, CV clinical events, and all-cause of mortality at Week 120. Additional exploratory endpoints include a change from baseline in cardiac imaging parameters, renal function, biomarkers, and PROs questionnaires and disease scores. An interim analysis on change from baseline in 6MWD is also planned at Week 60. All deaths and CV clinical events will be adjudicated by an independent, blinded Clinical Adjudication Committee, using predefined endpoint criteria.
Conclusions: Despite recent advances, there is still a need for more efficacious, safe and convenient treatment options for ATTR-CM. The ION-682884-CS2 is a large Phase 3 trial designed to evaluate the clinical efficacy and safety of AKCEA-TTR-LRx (ION-682884) compared to placebo for the treatment of ATTR-CM.
Falk:Ionis Pharmaceuticals: Consultancy. Gertz:Ionis/Akcea: Consultancy; Alnylam: Consultancy; Proclara: Membership on an entity's Board of Directors or advisory committees; Prothena Biosciences Inc: Consultancy; Celgene: Consultancy; Janssen: Consultancy; Spectrum: Consultancy, Research Funding; Annexon: Consultancy; Appellis: Consultancy; Amgen: Consultancy; Medscape: Consultancy, Speakers Bureau; Physicians Education Resource: Consultancy; Abbvie: Other: personal fees for Data Safety Monitoring board; Research to Practice: Consultancy; Teva: Speakers Bureau; Johnson and Johnson: Speakers Bureau; DAVA oncology: Speakers Bureau; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; i3Health: Other: Development of educational programs and materials; Springer Publishing: Patents & Royalties; Amyloidosis Foundation: Research Funding; International Waldenstrom Foundation: Research Funding. Benson:Ionis Pharmaceuticals: Research Funding. Buchele:Ionis Pharmaceuticals: Employment. Brambatti:Ionis Pharmaceuticals: Employment. Tsimikas:Ionis Pharmaceuticals: Employment. Viney:Ionis Pharmaceuticals: Employment. Tai:Ionis Pharmaceuticals: Employment. Monteiro:Ionis Pharmaceuticals: Employment. Yang:Ionis Pharmaceuticals: Employment. O'Dea:Akcea Therapeutics: Employment. Karwatowska-Prokopczuk:Akcea Therapeutics: Employment. Schneider:Ionis Pharmaceuticals: Employment. Geary:Ionis Pharmaceuticals: Employment. Monia:Ionis Pharmaceuticals: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal