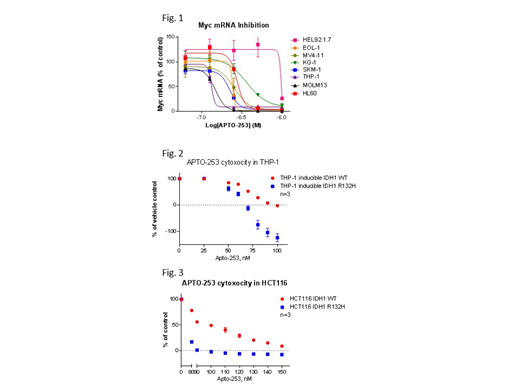
APTO-253 is a small molecule with a novel mechanism of action and potent antiproliferative activity against cell lines derived from a wide range of human malignancies. A Phase 1 study of APTO-253 was completed in patients with advanced solid tumors. It was well tolerated and produced evidence of antitumor activity in patients but did not cause myelosuppression even at the maximum tested dose. A phase 1a/b trial of APTO-253 in patients with relapsed/refractory acute leukemias (including AML) and high-risk MDS (NCT02267863) is currently underway. We previously reported that APTO-253 was converted intracellularly to a complex containing one molecule of iron and three molecules of APTO-253 [Fe(253)3]. Both APTO-253 and Fe(253)3 stabilized DNA quadruplex (G4) structures in the Myc promotor region to reduce the expression of Myc (Figure 1). Stabilization of G4 structures has been reported to stall replication forks and produce DNA strand breaks. Treatment of cancer cells with APTO-253 produced time and concentration-dependent γH2AX foci formation and DNA double strand breaks that have to be repaired by homologous recombination. Loss of either BRCA1 or BRCA2 function in multiple isogenic paired cell lines resulted in hypersensitivity to APTO-253 whose magnitude was similar to the effects of PARP inhibitors, olaparib. Thus, APTO-253 is a member of the limited repertoire of drugs which can exploit defects in homologous recombination and is of particular interest because it does not produce myelosuppression. The goal of this project was to identify synthetic lethal interactions in addition to BRCA1/2 deficiency that can guide combination drug studies. The normal function of the isocitrate dehydrogenase (IDH) enzymes is to catalyze the conversion of isocitrate to α-ketoglutarate (αKG) in the citric acid cycle. IDH1 mutations occur in more than 70% of low-grade gliomas, 20% of high-grade glioblastomas and with a frequency of about 10% in AML, cholangiocarcinomas, melanomas and chondrosarcomas. Additionally, mutations are also identified in IDH2 in about 4% of gliomas and 10% of AMLs. IDH1/2 alterations are heterozygous missense mutations that confer a neomorphic activity on the encoded enzyme such that they covert αKG to (R)-2-hydroxyglutarate [(R)-2HG]. (R)-2HG is an oncometabolite with pleiotropic effects on cell biology including chromatin methylation and cellular differentiation. It was reported that IDH1/2 mutations induce a homologous recombination defect that renders tumor cells exquisitely sensitive to PARP inhibitors.
We used 2 pairs of isogenic IDH1 wild type and mutant cell lines to test the hypothesis that cells carrying IDH1/2 mutations are hypersensitive to APTO-253. Induction of the expression of IDH1 R132H in THP-1 AML cells increased the 2-HG concentration ~150-fold whereas induction of wild-type IDH1 expression increased it only 2-fold (doi: 10.1038/nm.3788). THP-1 cells expressing the mutant IDH1 R132H were hypersensitive the treatment of APTO-253 compared to cells expressing the wild-type IDH1 (Figure 2). Comparison of wild-type and isogenic IDH1 R132H HCT116 cells disclosed a ~100-fold higher level of (R)-2-HG in the mutant cells (doi: 10.1126/scitranslmed.aal2463), and an increase in sensitivity to the cytotoxic effect of APTO-253 (Figure 3). These results are consistent with the observation that both APTO-253 and increased levels of 2-HG cause DNA damage that depends on homologous recombination for repair and that this favors a synthetic lethal interaction. We surmise that the ability of APTO-253 to markedly reduce the expression of Myc further contributes to the favorable interaction. This data further supports the conclusion that defects in homologous DNA repair can enhance the potency of APTO-253 and suggest the use of this non-myelosuppressive drug in both IDH1/2 mutant AML and solid tumors.
Zhang:Aptose Biosciences, Inc: Employment. Rice:Aptose Biosciences, Inc: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Howell:Aptose Biosciences, Inc: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal