Introduction:
Peri-transplant radiation (XRT) in Hodgkin Lymphoma (HL) patients undergoing autologous stem cell transplant (ASCT) has been associated with improved local control. However, this has not been well established with increased use of novel agents in peri-transplant setting.
Methods:
This was a retrospective analysis of HL patients who underwent their first ASCT between 2000 and 2018 at Karmanos Cancer Institute in Detroit, Michigan. Peri-transplant radiation was defined as XRT within 3-month window of autologous transplant. Novel agents included brentuximab, check-point inhibitors (CIs), and other targeted agents. The primary endpoint was to compare overall survival (OS) based on utilization of peri-transplant XRT and novel agents with standard salvage chemotherapy. Secondary endpoint was to assess the OS benefit of novel agent use in the post-transplant setting. Univariable and multivariable Cox proportional hazards regression models were fit to assess associations between OS and nine prior chosen predictors (bulky relapse, pulmonary or bone marrow relapse, extranodal disease, CT/PET response [CR vs less than CR], novel salvage [anytime], peri-transplant XRT, site of relapse [local vs systemic], maintenance brentuximab, relapse after initial diagnosis [>1 year vs ≤1 year]).
Results:
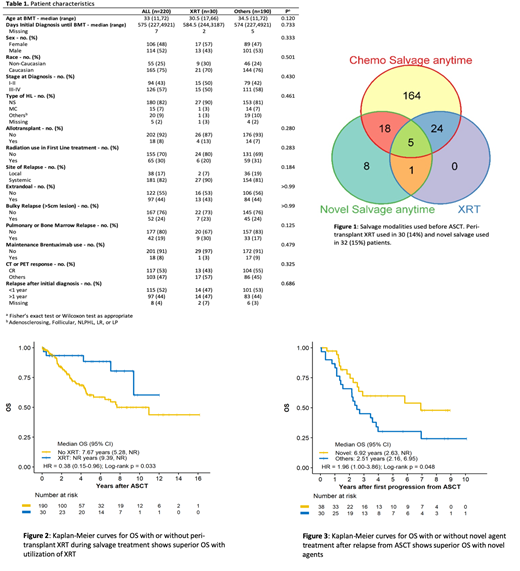
From 2000-2018, there were total of 220 patients who underwent ASCT for HL. Patients underwent ASCT median of 575 days after initial diagnosis. 65 (30%) patients had XRT during initial treatment and XRT was utilized less frequently in all patients (13% vs 35%) and stage I-II patients (29% vs 46%) who were diagnosed after 2010. Peri-transplant XRT was used in 30 (14%) patients and was utilized similarly in patients who had ASCT before or after 2010 (13% vs 14%) (Figure 1). There was no difference in baseline characteristics (bulky relapse, local vs systemic relapse, etc.) of patients who did or did not receive peri-transplant XRT (Table 1). Novel salvage was used in 32 (15%) patients at any time before transplant (Figure 1).
Median OS of all the patients who had ASCT was 11 years [7.6-NR] and PFS was 4.3 years [2.3-7.4]. Median OS of patients who had peri-transplant XRT was not reached [9.4-NR] compared to 7.7 [5.3-NR] years for those without peri-transplant XRT [HR 0.38; 95% CI 0.15-0.96; p=0.033] (Figure 2); on the contrary, there was no difference in PFS between the two groups. Median OS of patients who had novel salvage at any time was not reached [4.3-NR] compared to 11 [7.6-NR] years for those without novel salvage [HR 1.08; 95% CI 0.43-2.72; p=0.877]. Median OS of patients who had either novel salvage (anytime) or peri-transplant XRT was not reached [9.4-NR] compared to 11 years [6.5-NR] for those who had chemotherapy salvage only [HR 0.64; 95% CI 0.32-1.26; p=0.193]. In the multivariable analysis, less than CR to salvage therapy and lack of peri-transplant XRT use were associated with worse OS.
There were total of 85 (39%) patients who progressed after ASCT. Median OS for those patients was 3.5 years [2.3-8.8] and PFS was 1 year [0.8-1.5] after relapse from ASCT. The median OS for patients who received novel agents (brentuximab 61%, CI 14%) during first progression after ASCT was 6.9 years [2.3-NR] after relapse from ASCT compared to 2.9 years [2.3-NR] for those that did not [HR 1.47; 95% CI 0.72-3; p=. 288]. In addition, the median OS for patients who received novel agent at any time after transplant was 6.9 years [2.6-NR] after relapse from ASCT compared to 2.5 years [2.2-7] for those who did not [HR 1.96; 95% CI 1-3.86; p=0.048] (Figure 3). Lastly, OS after relapse from ASCT was better if patients progressing on novel agent post-transplant received another novel agent for their successive therapy rather than any other treatment [HR 4.87; 95% CI 1.14-20.8; p=0.018].
Conclusions:
We show that there was a lack of standardized patient selection for utilization of peri-transplant XRT and its use was similar before and after the novel agent era. Peri-transplant XRT was possibly used in patients who had residual disease or other high -risk features that could not be identified due to retrospective nature of this study and, despite this, use of peri-transplant XRT was associated with modest survival benefit. Surprisingly, use of novel agent at any time during salvage was not associated with survival benefit. Utilization of novel agents at any time after post-transplant progression was associated with OS benefit.
Deol:Kite: Other: Advisory board; Novartis: Other: Advisory board; Agios: Other: Advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal