[Background] Philadelphia chromosome positive (Ph+) leukemia is characterized by highly proliferative nature and clone instability that evokes the emergence of mutated clones, including BCR-ABL1 T315I mutated clone. Established evidence on the use of tyrosine kinase inhibitors (TKIs) after allogeneic hematopoietic stem cell transplantation (HSCT) is still lacking. The use of second-generation TKIs as a maintenance treatment after HSCT has been studied, and it is expected that their use would improve the prognosis by suppressing recurrence. The advent of ponatinib (PON), a potent inhibitor of tyrosine kinase including T315I mutated BCR-ABL1, is expected to improve clinical outcome of Ph+leukemia. However, there are few reports of a maintenance treatment using PON after HSCT.
[Methods] We retrospectively reviewed data of 13 patients (pts) who received PON for Ph+leukemia after HSCT while in hematological complete remission (CR) between April 1, 2016 and July 15, 2019. Prophylactic treatment (Pro) was defined as post-transplant administration of PON while in minimal residual disease (MRD) negative CR. Pre-emptive treatment (Pre) was defined as starting PON when the bcr-abl transcript was detected by either quantitative or nested qualitative PCR after HSCT. ABL1 mutation was analyzed through the direct sequencing method. Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events version 5.0. Overall survival (OS) was estimated using Kaplan-Meier method. Non-relapse mortality (NRM) and cumulative incidence of hematological relapse (CIR) were calculated using Gray's test. This study protocol was approved by the ethics committee of Tokyo Metropolitan Komagome Hospital.
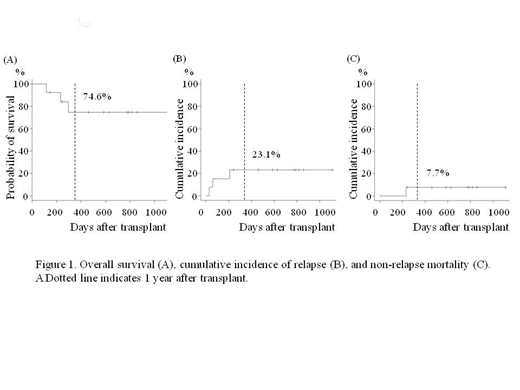
[Results] Underlying diseases were Ph+ALL in 8 pts (5 in CR, 3 in non-CR at HSCT), CML in 5 (all in second chronic phase). ABL1 mutations were analyzed in 12 pts and T315I mutation was detected in 4 pts with Ph+ALL and 2 with CML. Furthermore, compound mutations (CMs) in BCR-ABL1 were detected in 4 pts before HSCT. PON was used in 6 only after HSCT, and in 7 both before and after HSCT. During the median observation after HSCT of 584 days (range, 116-1,110) for survivors, no vascular occlusion event occurred. With regard to adverse events (AEs), grade 3 AEs occurred in 2 pts (15.4%) and no grade 4 AE was observed. Two had liver dysfunction and one of them discontinued PON due to grade 3 abnormalities in liver function tests. One suffered from grade 3 thrombocytopenia. Four had skin rashes lower than grade 3 that were indistinguishable from skin graft-versus-host disease, and all of them resolved through topical steroid therapy. Of all, 6 were in Pro group and 7 were in Pre group. The initial dose of PON was median 15mg (range 45mg/twice a week - 15mg/day) in Pro and median 30mg (range, 15-45mg) in Pre. The median days from HSCT to the start of PON was 107 days (range, 32-174) in Pro and 208 days (range, 50-364) in Pre. The median duration of PON treatment was 297 days (range, 20-699) in Pro and 188 days (range, 5-608) in Pre. At final observation in Pro group, 2 pts relapsed and died during the salvage therapy, 1 pt discontinued PON due to hepatic adverse event, and 3 pts were still on PON. Meanwhile, in Pre group, 5 pts achieved MRD negative CR after PON administration (1 pt also received donor lymphocyte infusion and stop PON due to liver dysfunction, 1 discontinued PON by the patient's request, and 3 of them were still on PON). One pt with CM relapsed but achieved CR through salvage therapy and 1 pt with low performance status (KPS 60) died at home of unknown cause six days after taking PON 30mg daily. For all the 13 pts receiving PON maintenance therapy, OS was 74.6% (95%CI; 39.8-91.1), CIR was 23.1% (95%CI; 5.1-48.5), and NRM was 7.7% (95%CI; 0.4-30.6) at 1 year after transplant (Figure 1). Two out of 4 pts with CMs (V299L/F317L and E255K/T315I/F317L) remains in MRD negative CR. The other 2 with CMs (E255K/T315I and D276G/T315I) had progressed to hematological relapse, suggesting the resistance to PON. In contrast, only one out of 9 without CMs relapsed on PON treatment.
[Conclusion] Our results suggested that post-transplant maintenance treatment using PON was tolerable in the majority of patients with Ph+leukemia, although the optimal dose or the initiation strategy (Pre or Pro) are still undetermined. Furthermore, some patients with T315I-inclusive CMs seemed to be resistant to PON. The longer observation in a larger cohort is warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal