Background:
AZA monotherapy has demonstrated improvement in OS in HR MDS, clinically meaningful and durable responses continue to be limited to a subset of patients (Silverman 2006). One obvious strategy is to develop doublet therapy with drugs that are effective monotherapy in HR MDS and can be administered effectively and safely in combination with AZA. Several agents have been combined with AZA in first line therapy for pts with HR-MDS achieving ORR and CR/PR rates that are comparable to AZA monotherapy (ORR 38% and CR/PR of 24%) (Ades ASH 2018; Sekeres JCO 2017). RAS and other signaling molecules in the Ras pathway are frequently mutated in HR MDS and are proposed to drive leukemic transformation (Takahashi 2013). Given that rigosertib interferes with the RAS-binding domain of RAF kinases and inhibits the RAS-RAF-MEK & the PI3Ks pathways (Athuluri-Divakar, Cell 2016), it is an attractive candidate for combination with AZA. Furthermore, in vitro combo of rigosertib with AZA synergistically inhibits growth and induces apoptosis of leukemic cells in a sequence-dependent fashion (Skiddan AACR 2006). We report updated results from a Phase II study in a subset of patients receiving oral rigosertib in combination with standard dose AZA as first line therapy for HR MDS.
Methods:
In the Phase II study 09-08, a total of 39 treatment-naïve HR MDS/RAEB-t patients received oral rigosertib in combination with standard dose AZA. Rigosertib was given at 840mg/day (560 mg in the morning & 280mg in the afternoon); or 1120mg/day (560 mg in the morning & 560mg in the afternoon or 840 mg in the morning and 280 mg in the afternoon) (Maniar ASH 2018). Responses were determined by 2006 IWG criteria including transfusion independence (56 days without PBC or PLT transfusions). Oral rigosertib was administered on D1-D21 of a 28D cycle. Parenteral AZA 75mg/m2/day was administered for 7days from D8. Patients were evaluable for response if they received 3 cycles of therapy.
Results:
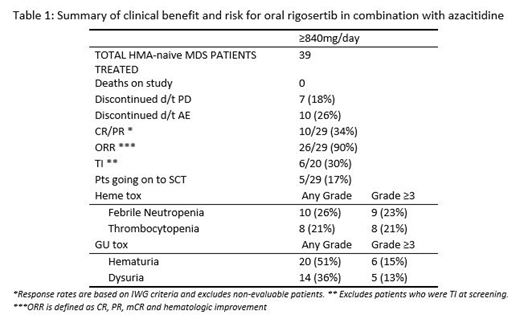
Median age of the pts was 64 years (42-90). IPSS-R score at study entry was 9 were Intermediate, 8 were High and 17 were Very High Risk (VHR). In total 20 pts were transfusion-dependent at study entry. CR/PR responses were observed across all IPSS-R cytogenetic prognostic subgroups: Very Poor 60%, Poor 25%, Intermediate 37.5% & Good 25%. CR/PR responses were also observed across all higher IPSS-R prognostic risk categories: Very High 42%, High 17% and Intermediate 25%.
A summary of clinical benefit/risk is provided in Table 1 below.
Median duration of response was 12.2 mos (0.1-24.2+) and median duration of treatment was 8 mos (1.3-27.3). Time to first and best responses was 1/4 mos. Four pts continue to respond to therapy. 10 AEs led to D/C: urinary tract pain (2), and 1 each for urinary retention, hematuria, hydronephrosis, osteolysis, cerebral haemorrhage, WBC count decreased, neutrophil count decreased, & abd pain. The most frequent AE in table 1, are similar to AEs reported for both rigosertib and AZA as monotherapies, & the GU toxicities were mitigated using specific management guidelines. The majority of MDS pts who experienced AEs ≥Grade 2 were successfully managed and continued to receive the doublet on study.
Conclusion:
The efficacy (90% ORR & 34% CR) and safety of oral rigosertib and AZA in combination is favorable as first line therapy in pts with HMA naïve HR MDS and are comparable to historical results for standard dose AZA monotherapy (ORR 38% and <20% CR). The transfusion independence (TI) of 30% in HR MDS pts is clinically meaningful and needs to be confirmed in a large randomized phase III study. Oral rigosertib in combination with AZA was well tolerated and has now been administered in repetitive cycles for more than two years. Rigosertib is attractive combination partner for AZA because of the oral formulation, non-overlapping toxicity, mechanism of action and synergy. Based on the efficacy results and favorable safety profile, a pivotal Phase III trial in higher-risk HMA naive MDS population is planned.
Navada:Onconova Therapeutics Inc: Research Funding. Garcia-Manero:Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. Atallah:Helsinn: Consultancy; Jazz: Consultancy; Helsinn: Consultancy; Jazz: Consultancy; Novartis: Consultancy; Takeda: Consultancy, Research Funding; Pfizer: Consultancy. Shammo:Otsuka: Consultancy, Honoraria; CTI Pharma: Research Funding; Novartis: Consultancy, Honoraria; Incyte: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Alexion: Consultancy, Honoraria, Research Funding, Speakers Bureau; Onconova: Research Funding; Apellis: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Speakers Bureau; Astex Pharma: Research Funding. Griffiths:Abbvie, Inc.: Consultancy, PI on a clinical trial; Celgene, Inc: Consultancy, Research Funding; Genentech, Inc.: Research Funding; Novartis Inc.: Consultancy; Novartis Inc.: Consultancy; Genentech, Inc.: Research Funding; Boston Scientific: Consultancy; Boston Scientific: Consultancy; Persimmune: Consultancy; Persimmune: Consultancy; New Link Genetics: Consultancy; New Link Genetics: Consultancy; Astex Phramaceuticals/Otsuka Pharmaceuticals: Consultancy, Research Funding; Astex Phramaceuticals/Otsuka Pharmaceuticals: Consultancy, Research Funding; Partner Therapeutics: Consultancy; Appelis Pharmaceuticals: Other: PI on a clinical trial; Onconova Therapeutics: Other: PI on a clinical trial; Appelis Pharmaceuticals: Other: PI on a clinical trial; Onconova Therapeutics: Other: PI on a clinical trial; Abbvie, Inc.: Consultancy; Partner Therapeutics: Consultancy; Celgene, Inc: Consultancy, Research Funding. Khaled:Alexion: Consultancy, Speakers Bureau; Omeros: Consultancy; Daiichi Sankyo: Other: Travel support. Adesanya:Onconova Therapeutics, Inc.: Employment. Zbyszewski:Onconova Therapeutics, Inc.: Employment. Woodman:Onconova Therapeutics, Inc.: Employment. Fenaux:Jazz: Honoraria, Research Funding; Aprea: Research Funding; Celgene Corporation: Honoraria, Research Funding; Astex: Honoraria, Research Funding. Silverman:Medimmune: Research Funding; Celgene: Research Funding; Onconova Therapeutics Inc: Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal