Purpose
Autologous hematopoietic stem cell transplantation is commonly used as treatment for patients with Multiple Myeloma (MM) or Non-Hodgkin Lymphoma (NHL). The mobilization and collection of peripheral blood progenitor cells (PBPC) to reach the required cell dose for transplantation is problematic for some patients when standard mobilization agents such as G-CSF, alone or combined with chemotherapy, are used. These patients are considered poor mobilizers with < 20 CD34+ cells /μl on the planned day of collection and the optimal strategy to collect a sufficient number of PBPC in poor mobilizers is still not fully known. Plerixafor, a CXCR4 inhibitor, when added to the standard mobilization regimen, has been shown to improve mobilization and increase the probability of collecting a PBPC graft > 2 X106CD34 /kg in standard mobilizers. In our center, Plerixafor became available in October 2012. However, how best to use plerixafor to improve care for poor mobilizers while ensuring the most cost-effective use of health care resources for this patient population remains an important question. To begin answering this question, we evaluated the impact of plerixafor usage in our population of poor mobilizers in our institution using a historical cohort for comparison.
Material and method
The population of poor mobilizers in the setting of MM or NHL (defined as < 20 CD34 / μl on the planned day of collection) between January 2009 and December 2017 at Hôpital de l'Enfant Jesus (HEJ) du CHU de Québec was retrospectively studied and analyzed. To ensure that all poor mobilizers were included, we screened all flow cytometry CD34+ cell count results < 50 /μl on the planned day of collection and reviewed all the charts of patients with < 20 CD34 / μl regardless of whether they were collected or not. This chart review allowed a comprehensive description of the characteristics of our poor mobilizers, a comparison of the capacity to collect an adequate PBPC with or without plerixafor and to evaluate its impact on different variable with regard to PBPC collection and subsequent engraftment. The data were compiled in an ACCESS database using a retrospective review of digital patient charts. The data was analyzed by the Centre de Recherche du CHU de Quebec (CRCHUQ). Data were stratified by disease type and CD34 level on the planned day of PBPC collection.
Results
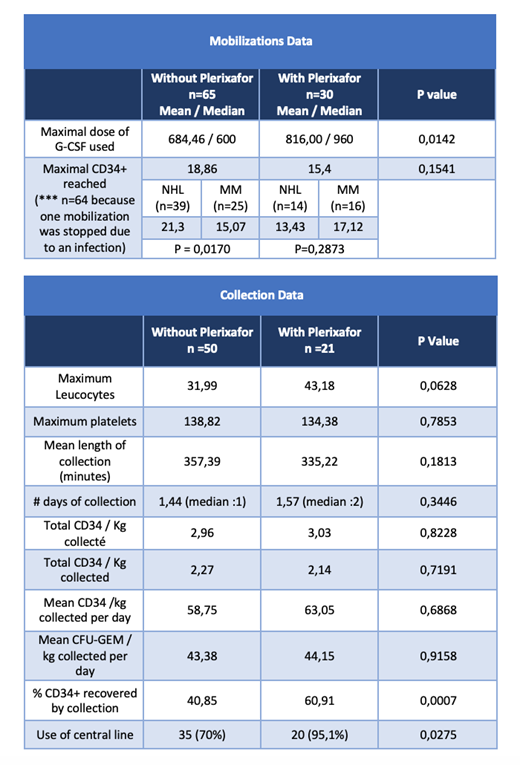
207 patient charts were reviewed; 70 poor mobilizers with 95 mobilizations attempts, 30 of them using plerixafor, were included. The baseline characteristics of both groups were similar as well as the length of hospitalization for transplant and relapse free survival at one year. The addition of Plerixafor to our management of poor mobilizers did not significantly increase the proportion of patients proceeding to autologous PBPC transplant (80,4 vs 75% p=0,7599). During the mobilization attempts, the maximal CD34 /μl reached was similar in both groups (18,86 vs 15,4 p=0,1541). Plerixafor was associated with a superior collection efficiency (% of CD34 collected) (40,85 vs 60,91% p=0,007), but this can be explained by a greater use of central venous catheters for collection in this group (70 vs 95,1% p = 0,0275). Data stratified by initial diagnosis and CD34 level on the planned day of PBPC collection (<10 c/μl versus [10c/μl - 20c /μl [) showed the same tendency. The collection duration was not significantly reduced in the plerixafor group, but there was a trend for a shorter mean duration of 22 minutes also explained by a greater use of central venous catheters in this group. The preplanned subgroups analyses have shown the same trend when stratified by diagnosis, with a reduction of 53 minutes in the multiple myeloma subgroup with a greater total CFU-GEM / kg per collect (46,35 vs 70,55 p=0,0163).
Conclusion
The use of plerixafor at HEJ did not improve significantly the proportion of poor mobilizers reaching an adequate PBPC graft and did not improve transplant outcomes compared to a historical control. Better strategies are needed to improve care for true poor mobilizers. The optimal use of plerixafor needs to be further studied.
Blais-Normandin:Sanofi: Other: unrestricted research grant. Laroche:Sanofi: Other: unrestricted research grant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal