Introduction
Multiple Myeloma (MM) is a plasma cell dyscrasia characterized by neoplastic proliferation of plasma cells. Despite the recent advances in disease management, it still contributes to significant morbidity and mortality, thus highlighting the unmet need for new treatment options. B-cell maturation antigen (BCMA) is a transmembrane glycoprotein in the tumor necrosis factor receptor superfamily 17 and has been shown to have high expression on plasma cells. Preclinical studies in MM have shown significant efficacy for BCMA targeted immunotherapies, thus providing a potential therapeutic option to overcome the obstacle of myeloma relapse and recurrence. In this comprehensive review, we have reported the role of anti-BCMA therapies in the treatment of relapse and refractory Multiple Myeloma (RRMM).
Methods
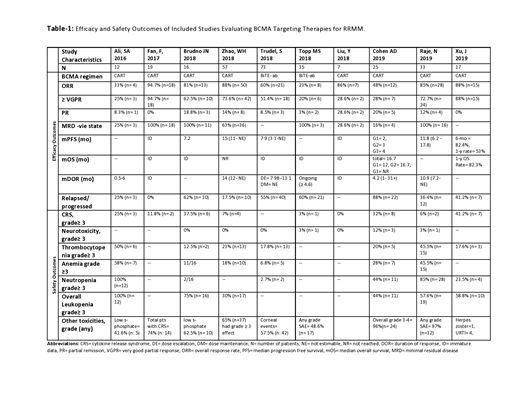
Systematic review was performed according to PRISMA statement. PubMed, Ovid Embase, Web of Science, Cochrane Library & Clinicaltrails.gov were searched, without any filters, using the medical search terms (MeSH) "Multiple Myeloma" AND "B Cell Maturation Antigen". Most recent reports of original phase I/II/III studies reporting safety and efficacy measures of anti-BCMA immunotherapies in RRMM were considered for inclusion. After a detailed screening of 854 studies, 10 phase-1 studies (Ali, SA. 2016, Fan, F. 2017, Brudno, JN. 2018, Zhao, WH. 2018, Trudel, S. 2018, Topp, MS. 2018, Liu, Y. 2018, Cohen AD. 2019, Raje, N. 2019 & Xu, J. 2019) were considered for inclusion.
Results (Table-1)
A total of 294 patients with RRMM who had received a median range of 3-7 previous lines of therapies were included. Out of these, 36.7% (n= 108) patients received bispecific T-cell engager (BiTE) antibodies against BCMA antigen while rest of patients received anti-BCMA chimeric antigen receptor T-cell (CART) therapy. Patients with CART therapy received autologous derived in-vitro modified stem cells. These patients subsequently developed higher incidence of grade 3-4 cytopenias owing to myeloablation with fludarabine and cyclophosphamide. The dosage ranges for both therapies were heterogenous owing to phase I dose escalation designs. All responses were evaluated according to IMWG response criteria. The cumulative overall response rate (ORR) for studied population was 59.86% (n= 176), while individual ORR was 26.85% (n= 29) for BiTE therapy and 79% (n=147) for CART therapy. Majority of responders (82%, n= 145) received ≥VGPR (very good partial response) status. But relapse/progression of disease after achieving response was a significant issue as 42.5% (n= 125) patients relapsed or progressed and developed BCMA negative myeloma cells in their bone marrow. The progression free survival and survival data is premature at this stage and requires further elaboration in phase II/III clinical trials. Toxicity profile was consistent with use of myeloablation and with the standard toxicity profile seen in other B-cell malignancies except for the incidence of grade ≥ neuropathy which was not much common (1.7%, n= 5). Regarding the grade ≥ 3 cytopenias, incidence of neutropenia was 20% (n=59), anemia 21.4% (n=63), thrombocytopenia 19.4% (n= 57). Grade ≥ 3 cytokine release syndrome (CRS) seen in 11.2% (n= 33) patients which responded well to Tocilizumab and corticosteroids except for death in two cases. All studies showed a significant correlation of CART cell dose and myeloma tumor burden with intensity of cytokine release syndrome (CRS). In each dose escalation trial, sustained and better responses were achieved at higher doses with a parallel enhanced incidence of grade 3-4 toxicities. The responses were not found to be influenced by previous lines of therapy, and patients with higher risk cytogenetics showed a reduced response compared to patients with standard risk cytogenetics in all studies.
Conclusion
BCMA targeted immunotherapies provide a promising option for highly refractory multiple myeloma patients. But small patient population and phase-I design limit the projection of results to real world setting. Furthermore, relapse/progression risk needs to be weighed against the significant toxicity profile. So, more controlled clinical trials with longer follow up periods are needed to delineate the efficacy and survival outcomes of this therapeutic option.
Anwer:In-Cyte: Speakers Bureau; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal