Introduction:
About 60% newly diagnosed multiple myeloma (NDMM) cases are above age of 60 years. The paucity of studies exclusively targeting management of frail patients has led to persistence of therapeutic uncertainties. With data on more than ten thousand patients, purpose of this review is to summarize the available therapeutic options with emphasis on recent advances for treatment of frail and elderly, transplant ineligible NDMM patients.
Methods:
We performed a comprehensive literature search on June 1st, 2019 on PubMed, Cochrane Library and ClinicalTrials.gov. We used the MeSH terms: 'Multiple Myeloma' and 'Frail Elderly', with associated entry words. Search yielded 71 studies regarding our topic of interest. Following PRISMA guidelines and subsequent screening by two reviewers, we shortlisted 19 ongoing/completed studies (n=10297) and included data from these studies in our systematic review.
Results:
Two/Three Drug regimens:
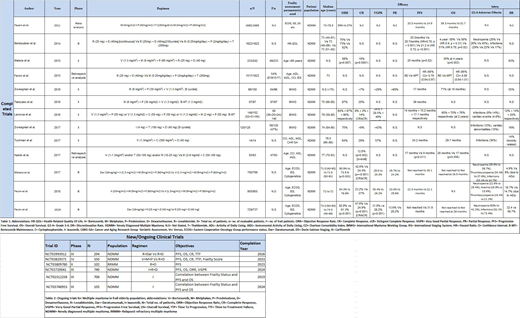
Among the two drug regimens [Table 1], Lenalidomide (R) and Dexamethasone (D) (RD) combination has been most widely studied (n=1445). RD yielded objective response rate (ORR) of 81.3%, complete response (CR) or above of 24.9% and progression free survival (PFS) of 31.9 months in a phase III trial (Usmani, 2019) (n=369). Facon et al. (2019) [n=368] used Daratumumab (Dara)+R+D (DaraRD) which exhibited the best response overall with an ORR of 92.9%, CR of 47.6%, VGPR of 31.8% while the PFS was not reached till study end point. However, >grade 3 neutropenia developed in 50% patients.
Three-drug regimen of Bortezomib(V)+Melphalan(M)+Prednisolone(P) (VMP) has been the most widely studied regimen (n=1059) in four phase II/III clinical trials. In a phase II trial (Kizaki, 2016) (n=87), VMP yielded a PFS of 36 months and CR of 25%. In a Phase II trial by Larocca et al (2016, n=148), 3 cohorts (VP, V+ Cyclophosphamide (C) +P and VMP respectively) were studied. Best response was achieved by VMP with ORR of 86%, PFS of 17.1 months and CR of 14%, compared with VCP (ORR=67%, PFS=15.2 months and CR=2%) and VP (ORR=64%, PFS=14 months, CR=8%). However, the discontinuation rate (DR) due to AEs for VMP was relatively high (20%).
A phase III trial (San-Miguel, 2018) (n=955) compared Carfilzomib(K)+M+P (KMP) against VMP. Median PFS was found to be 22.3 months with KMP Vs 22.1 months with VMP. Grade ≥3 AE rates were 74.7% for KMP and 76.2% for VMP. Thus, the results showed no significant difference between both regimens.
Thalidomide (T) has also been used in three drug combinations in two phase II/III trials (n=667). Ixazomib(I)+T+D (ITD) in a phase II trial (Abildgaard, 2017) (n=120) revealed an ORR of 75% compared to ORR of 62% in a phase III trial (Benboubker et al, 2014) (n=547) using MPT. Notable >grade 3 AEs with ITD were infections (15%) and cardiac abnormalities (10%) while with MPT were >grade 3 neutropenia (45%) and infections (17%).
A retrospective analysis by Facon et al. (2015, n=1517) comparing RD Vs MPT demonstrated that RD reduced the risk of progression or death by 21% compared to MPT in frail patients.
2. Four Drug Regimens:
Four drug regimens have also been used in transplant-ineligible patients in two phase II/III trials (n=583). Mateos et al. (2015, n=233) conducted a phase II trial in which patients were treated with VMP+RD (VMPRD). 49 frail patients based on Age >80 years (IMWG criteria) had ORR of 68%, PFS of 25 months and CR of 10%, with a DR of 63% due to toxicity or informed consent withdrawal. However, in the ALCYONE trial (San-Miguel, 2017) (n=350), use of Dara+VMP (DaraVMP) resulted in ORR of 90.9%, ≥CR of 42.6%, VGPR of 28.6% and PFS was not reached till study end point. Furthermore, the DR due to AEs for DaraVMP was also lesser (4.9%).
Various trials [Table 2] are being conducted to establish correlation of frailty scores with parameters of efficacy.
Conclusion:
Management of frail and elderly NDMM patients is challenging as there is need to individualize therapy for this group. Novel agents such as lenalidomide, bortezomib and daratumumab have shown promising efficacy when used as combination therapies with other conventional agents. Intensity of treatment and efficacy goals should be tailored to the functional capacity and tolerance of each individual patient. There is need for focused clinical trials for this group in terms of greater recruitment into clinical trials to establish better correlation between frailty status and efficacy, and consolidating evidence for improved patient care.
Anwer:In-Cyte: Speakers Bureau; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal