Introduction:
Melphalan causes cross linkage between DNA, causes cytotoxicity in both dividing and non-dividing tumor cells. Our objective is to analyze and summarize the published literature for the efficacy of melphalan based regimen used for the treatment of newly diagnosed Amyloidosis (ND-AL).
Methods:
We performed a comprehensive literature search on articles following PRISMA guidelines. Beginning with articles published after June 2006, we used databases like PubMed, Embase, Clinicaltrials.gov, Cochrane Library and Web of Science. Total 649 articles were identified initially and after detailed screening, we finalized 10 studies involving 616 ND-AL patients.
Results:
Melphalan (M), Bortezomib (V) and dexamethasone (d)/prednisone (p):
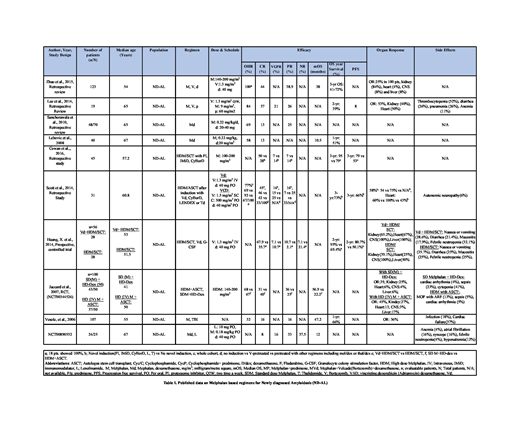
A retrospective study by Zhao et al., included 123 ND-AL patients (pts) were given M, V, and d. Overall hematological response (OHR) was 100% with complete response (CR) in 44% and partial response (PR) in 38.9% pts. Median overall survival (mOS) was 38 months (mo) and 3-yr survival ranged from 41-72%. Organ response (OR) was 25%. In a study by Lee et al., with 19 pts who received M, V, and p demonstrated OHR of 84% (Table 1).
Melphalan (M) and dexamethasone (d):
Sanchorawala et al. (n=70) reported patients treated with M and d showed OHR of 69%, with CR in 13% and PR in 25%. Similarly, a study by Lebovic et al. reported 40 pts who were given M, d. OHR was 58% and 13% pts showed CR (Table 1).
High dose Melphalan/Stem Cell Transplant (HDM/SCT) with and without induction:
In study by Cowan et al., (n=45) pts in group A (n=21) were given novel induction using agents like protease inhibitor (PI), cyclophosphamide, bortezomib and dexamethasone (CyBorD), Lenalidomide (L), dexamethasone (d) prior to high-dose melphalan (HDM). CR was observed in 50%, VGPR in 7% and PR in 7% pts. Group B (n=24) pts were given frontline HDM/SCT upfront. CR was observed in 28%, VGPR in 14% and PR in 14% pts. In a study by Scott et. al., 31 pts were categorized in 3 groups who received HDCT either with no induction, induction with V-based regimen and induction with other regimens including lenalidomide/dexamethasone (len/dex), melphalan/dexamethasone (mel/dex) and thalidomide/dexamethasone (Thal/dex). OHR in mel/dex cohort (n=3) was 67% (Table 1). In a study by Huang, X. et al., 56 pts were divided in two groups of 28 pts each. Pts in group A received Vd+HDM/SCT demonstrated CR in 67.9%, VGPR in 7.1%, PR in 10 .7 % and no response (NR) in 7.1 % pts. In group B, pts received with HDM/SCT upfront demonstrated CR in 35.7%, VGPR in 10.7%, PR in 2.1 % and no response NR in 21.4 % pts (Table 1).
Randomized Standard dose Melphalan (SDM) versus HDM:
In a study by Jaccard et al., there were two groups. The OHR was 68% in group A pts who were given SDM and high-dose dexamethasone (HD-dex) with CR in 31% and PR in 36% pts. The OHR was 67% in group B pts who were given HDM+ASCT with CR in 40% and PR in 25% pts (Table 1).
Melphalan with Total body irradiaton (TBI):
Vesole et al., reported 107 pts who were given M and TBI. OHR was 32% with CR in 16% and PR in 16% pts. mOS was 47.2 mo (Table 1).
Melphalan (M), dexamethasone (d), Lenalidomide (L):
In a clinical trial (NCT00890552) involving 25 pts M, d, and lenalidomide were give. CR, VGPR and PR were observed in 8%, 16% and 33%. 37.5% pts showed no-response (Table 1).
Conclusion:
Despite heterogeneity in the AL patient population and various regimens used in published literature, melphalan based regimens are very effective for treatment. Induction regimens and supportive care have evolved over the years. Novel combination regimens used for induction followed by HD-Melphalan consolidation along with careful selection of patients for high dose chemotherapy consolidation and stem cell transplantation in routine clinical practice is the best approach for personalized therapy selection for AL amyloidosis. Cytopenias of three cell lines are the major side effects reported with Mel therapy. Just like melphalan use for treatment of multiple myeloma in novel combination regimens, future randomized prospective trials are needed to better understand the efficacy and safety profile of melphalan based newer combination regimens for AL amyloidosis treatment.
Anwer:In-Cyte: Speakers Bureau; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal