Background
Triplet combination therapies are the preferred treatment choice for RRMM, however, high-cost-high-cost drug combinations pose significant barriers for health technology assessments (HTA) and access in many public healthcare systems, including the UK.
Selinexor is a first-in-class Selective Inhibitor of Nuclear Export (SINE) compound that binds and inactivates Exportin 1 (XPO1), thereby forcing the nuclear retention of key tumour suppressor proteins (TSPs), leading to MM cell death. Recently, the FDA has granted accelerated approval to selinexor for MM on the basis of results from the STORM trial, which tested selinexor in doublet combination with dexamethasone in RRMM. Several trials exploring triplet combinations are ongoing, but most are with high-cost combination partners. In the Myeloma UK Twelve (MUKTwelve) trial Selinexor is combined with low-dose cyclophosphamide and prednisolone in a randomized phase II design, exploring a triplet regimen that could potentially be more accessible for wider patient population.
Study design and methods
MUKtwelve is a randomized, controlled, open, parallel group, multi-centre phase II trial designed to evaluate clinical efficacy of selinexor in combination with cyclophosphamide and prednisolone (SCP) in patients with RRMM (ISRCTN15028850). The primary objective is to determine whether the addition of selinexor to cyclophosphamide and prednisolone (CP) may lead to increased progression free survival (PFS) compared to historic CP data. A calibration arm of patients will receive CP alone, and will be used to evaluate the validity of the outcome in the experimental arm in comparison to historical control data. Participants who experience disease progression on the CP arm may, if deemed eligible receive SCP.
Eligible participants are those with RRMM who have received ≥ 2 prior anti-myeloma treatments including a proteasome inhibitor and lenalidomide. Entry criteria are inclusive to allow for a real-world RRMM population, including GFR >=20, no limitation regarding pre-existing polyneuropathy and allowance of growth factor and transfusion support.
A maximum of 60 participants will be recruited (45 participants in the SCP arm, and 15 in the CP arm) from 10 UK NHS hospitals. Participants are randomized on a 3:1 basis to receive either SCP or CP. The trial opened on 20th July 2018 and as of 23rd July 2019 16 patients have been randomized.
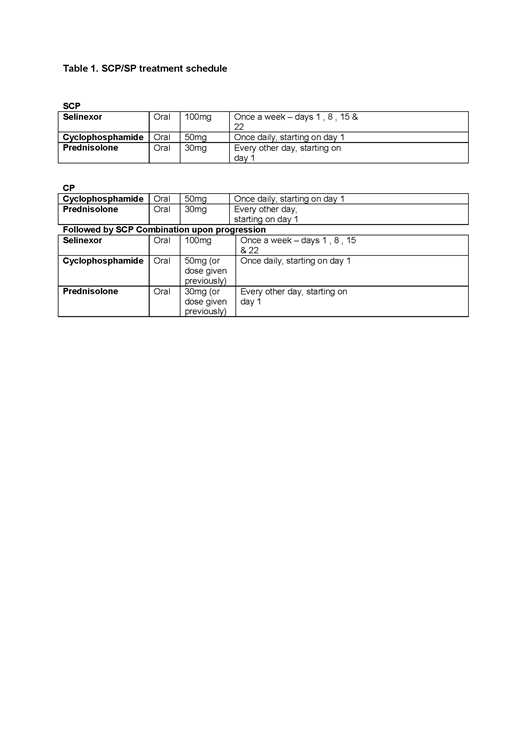
Trial treatment is as shown in Table 1 (28 day cycle). Metronomic, continuous doses of cyclophosphamide and intermittent doses of prednisolone in conjuncture with proactive, prophylactic side effect management, in particular nausea and kachexia, have been chosen to make the trial more accessible for a wider RRMM population.
The primary endpoint is proportion of patients alive and progression-free at 6 months (PFS6m). Secondary endpoints include response, response duration, safety and toxicity, PFS and treatment compliance. A three outcome statistical design is used based on the SCP arm only. The trial is designed to test the hypothesis PFS 6m ≤0.28 vs. PFS 6m ≥0.44 (equivalent to median PFS 3.3 vs. 5.1 months). With 90% power, 1-sided 10% significance level, a total of 45 patients are required in the SCP arm. Cut-off values and conclusions for the three outcome design:
≤14/45 patients PFS6m, SCP does not warrant further study
≥17/45 patients PFS6m, sufficient evidence for further study of SCP
15 or 16/45 patients PFS6m, decision uncertain and can be based upon other secondary endpoints.
This approach provides more realistic decision-making compared to those where success of failure is hinged on a difference of a single primary endpoint event.
Discussion
The MUKtwelve trial is the first trial to assess the clinical efficacy of selinexor with low-dose cyclophosphamide and prednisolone in RRMM, a triplet regimen that, if found to be effective, is likely to be accessible to a wider patient population.
Brown:Amgen, Celgene, Janssen, Karyopharm: Other: Research funding to Institution. Hall:Celgene, Amgen, Janssen, Karyopharm: Other: Research funding to Institution. Kendall:Karyopharm: Other: Research funding to Institution. Ingleson:Karyopharm: Other: Research funding to Institution. Flanagan:Amgen, Celgene, Janssen, Karyopharm: Other: Research funding to Institution. Auner:Karyopharm: Consultancy; Takeda: Consultancy; Amgen: Other: Consultancy and Research Funding. Boyd:Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Garg:Janssen: Honoraria; Janssen, Takeda, Novartis: Other: Travel expenses; Novartis, Janssen: Research Funding. Kaiser:Celgene, Janssen: Research Funding; Takeda, Janssen, Celgene, Amgen: Honoraria, Other: Travel Expenses; Abbvie, Celgene, Takeda, Janssen, Amgen, Abbvie, Karyopharm: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal