Fractalkine (CX3CL1) is a chemokine constitutively expressed in many hematopoietic and non-hematopoietic tissues (Bazan J.F. et al., Nature, 1997). It mediates different biological activities by binding to its receptor CX3CR1, expressed on different cell types such as natural killer (NK) cells, monocytes, cytotoxic effector T cells and B cells. The CX3CL1/CX3CR1 axis is involved in the pathogenesis of several cancers including various B-cell malignancies (Ferretti E. et al.,Mediators Inflamm, 2014). CX3CR1 was found both at the mRNA and protein levels in several subtypes of lymphoma (Andreasson, U. et al.,Cancer Lett, 2008).
In this study, we analysed the expression of the fractalkine receptor (CX3CR1) in peripheral blood mononuclear cells (PBMC) of chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) patients (pts) with active disease (n=22), pts with newly diagnosed diffuse large B-cell lymphoma (DLBCL) (n=21) as well as classical Hodgkin lymphoma (cHL) (n=19) and compared with healthy donors (n=14). PBMC populations were assessed by flow-cytometry including T cells, NK cells and monocytes. Statistical comparisons between groups were done using the non-parametric Mann-Whitney U test.
CLL/SLL pts had a median age of 75 years with equal sex distribution. Thirteen pts had high-risk and 9 intermediate-risk disease according to the modified Rai staging system; 1/21 had 17p deletion. Median lymphocyte count was 103 x 109/L (range 1.5-312). Of the 19 cHL pts, 12 had limited (stage 1-2A) and 7 had advanced (stage 2B-4) disease. Of the 21 DLBCL pts, 10 had stage 1, 4 had stage 2, 2 had stage 3 and 5 had stage 4 disease.
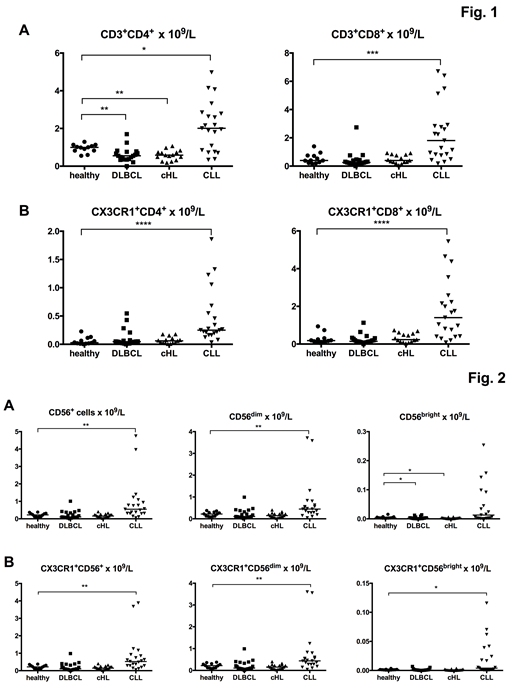
CLL/SLL pts had significantly higher absolute number of CD4+and CD8+ T cells compared to controls, DLBCL and cHL patients (p=0.02, p=0.0001 and p<0.0001 for CD4+ T cells and p=0.0007, p<0.0001 and p=0.0002 for CD8+ T cells, respectively). DLBCL and cHL pts had lower CD4+ numbers compared to controls (p<0.005), while no difference was observed for CD8+ cells (Fig. 1A). The absolute numbers of CD4+ and CD8+ cells expressing the CX3CR1 were also significantly higher in CLL pts compared to controls and the other patient groups (p≤0.0001 for all comparisons). No significant difference was observed between either DLBCL or cHL pts and controls (Fig. 1B).
Both the CD56bright and the CD56dim NK cell populations were also studied. CLL pts were found to have a significantly higher numbers of NK cells (p=0.001) and CD56dim NK cells (p=0.0027) compared to controls, while no difference was found for the other patient groups. No difference was observed between CLL pts and controls with regard to the CD56bright NK cells, which, however, were lower in both DLBCL and cHL pts than in controls (p<0.05) (Fig. 2A). The absolute numbers of NK cells expressing CX3CR1 was also significantly higher in CLL compared to controls (p=0.002). This was observed in both the CD56dim (p=0.004) and the CD56bright (p=0.04) subpopulations. In the other patient subgroups, no significant difference was observed with the exception of CX3CR1+CD56bright NK cells, which were lower in both DLBCL and cHL pts compared to controls (Fig. 2B).
The median monocyte count was not significantly different among the patient groups or controls. Monocyte sub-populations were then defined by flow-cytometry: classical (CD14highCD16low), intermediate (CD14highCD16high) and non-classical (CD14lowCD16high). The median percentage of monocytes expressing CX3CR1 was >95% in all the patient groups and did not significantly differ from controls. No significant difference was observed for the monocyte subpopulations either.
In conclusion, CLL/SLL pts with active disease have significantly higher numbers of T and NK cells expressing the fractalkine receptor (CX3CR1) compared to both controls and pts with DLBCL and cHL. No difference was observed for the monocytes. CX3CR1 might be a therapeutic target in CLL/SLL. Small molecule inhibitors of this axis are in clinical development.
Palma:Takeda: Research Funding. Olin:Kancera AB: Employment, Equity Ownership. Schultz:Kancera AB: Employment, Equity Ownership. Mellstedt:Kancera AB: Consultancy, Equity Ownership, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Österborg:Kancera AB: Research Funding; Abbvie: Research Funding; Janssen: Research Funding; BeiGene: Research Funding; Gilead: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal