Background:In 2016 WHO recognized the provisional entity refractory anemia with ring sideroblasts and thrombocytosisas a distinct entity and renamed it myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis (MDS/MPN-RS-T). The disease presents hallmarks of both myelodysplastic and myeloproliferative features. Although the diagnostic criteria are well defined and there are several studies of the molecular landscape of the disease, the treatment regimen of these patients remains inconsistent. Due to lack of clinical trials, treatment options are chosen either on MDS- or MPN-based regimens and individual preferences such as patients characteristics and clinical presentation. Prevention of thromboembolic events is a reasonable goal. However, the use of platelet-lowering drugs is still not well studied and treatment related anemia leading to transfusions with accelerated iron overload limits a general use of cytoreductive treatment. To avoid worsening of the anemia while efficiently reducing thrombocytosis we treated 3 patients with pegylated interferon (pIFN) and provide insight in JAK2-allele burden measurement and additional mutations obtained with next generation sequencing (NGS).
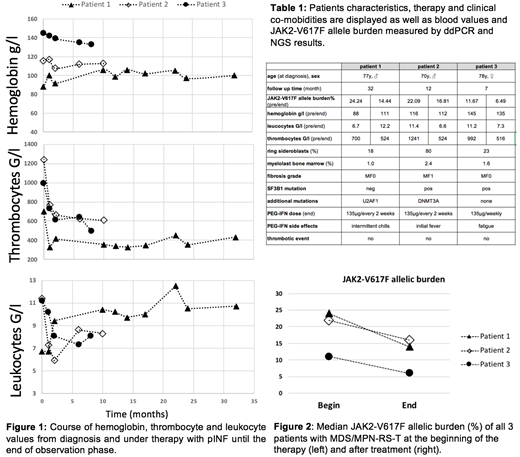
Results:Three patients with diagnosis of MDS/MPN-RS-T were treated with 135µg pIFN during an observation period between 8 and 34 months. All 3 patients had a JAK2V617F mutation, >15% ringsideroblasts and a thrombocyte count above 600G/l. In the additional NGS analysis, two patients showed a mutation in the SF3B1 gene, one with an additional DNMT3A mutation. In the third case, NGS results revealed a mutation in the splicing factor U2AFI, which is a rare finding in patients with MDS/MPN-RS-T. JAK2-allelel burden was obtained at the beginning and showed a decrease of a mean value of 38% as measured by digital droplet PCR. Anemia was present at initial diagnosis in two patients. During observation period, none of the patient became transfusion dependent, in one case even an improvement in hemoglobin levels was achieved. Side effects of the treatment were only minimal and no thromboembolic as well as major bleeding event occurred under additional acetylsalicylic acid medication.
Conclusion: In summary we show that in patients with MDS/MPN-RS-T a therapy with pIFN achieved rapid decrease of thrombocyte values without lowering hemoglobin levels at a maximal dose of 135µg per week. JAK2-allele burden reduction and a low side effect profile further demonstrate the value of this treatment option for these often elderly patients. However, larger studies are needed to confirm our data.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal