Background:
The myelodysplastic syndromes (MDS) represent heterogeneous disorders with varied clinical courses. The major prognostic tool in MDS is the IPSS-R, which helps estimate survival outcome and estimate risk for AML transformation. In low or intermediate risk pts, the IPSS-R has shortcomings; in these pts, the development of transfusion dependent anemia is the major disease associated complication, and this is not addressed by IPSS-R stratification. Previous studies have indicated that aberrancies detected by flow cytometry can risk stratify pts with MDS. The purpose of this study was to determine whether detection of neoplastic-specific blast aberrancies in pts diagnosed with low or intermediate risk MDS can identify pts at higher risk for transfusion dependent anemia after MDS diagnosis.
Methods:
We performed a retrospective chart review on MDS patients initially diagnosed at our institution between 1/2010 and 12/31/2017. Patients with low/intermediate risk by IPSS-R were identified. Flow cytometry findings on initial diagnostic BM biopsies performed at our institution only were reviewed. Flow cytometry (4- and 8-color) was performed on bone marrow aspirates for the following antigens: CD3, CD7, CD11b, CD13, CD14, CD15, CD19, CD20, CD33, CD34, CD36, CD38, CD45, CD56, CD64, CD117, and HLA-DR using FACS Calibur or FACSCanto II flow cytometers. Myeloblasts were identified by cluster analysis, as previously described (Am J Clin Pathol. 2010 Nov; 134(5):749-61), and compared to 20 control cases. Blast aberrancies were defined as an immunophenotypic difference of > ¼ log compared to the blasts in the controls. Neoplasia-specific blast aberrancies were defined as: expression of CD7, CD11b, CD15, and/or CD56 and/or under expression of CD38 and CD45. We estimated probability of transfusion dependent anemia using Kaplan Meier product limit method and compared survival curves using log-rank test. Analyses were performed using Stata v12.0.
Results:
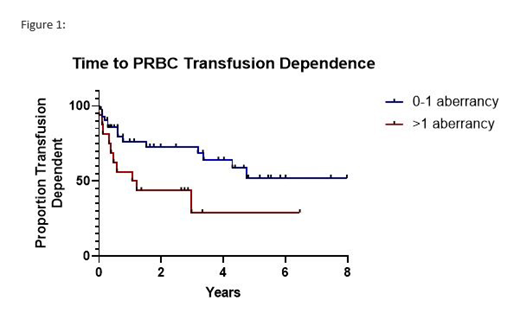
A total of 63 patients were identified, with median age of 68 years (range 31-89 years). Median hemoglobin (Hg) at diagnosis was 9.8 (range 5.2-15.3). Cytogenetic risk categories were very good, good, intermediate and poor in 3%, 71% 16%, and 10% respectively. IPSS-R category was very low or low in 70% (44 pts), and intermediate in 30% (19 pts). The presence of blast aberrancies was similar in proportion among low risk patients (61%, n=27) compared to intermediate risk patients (68%, n=13). Overall, the presence of only one blast aberrancy, whether neoplasia-specific or not, did not significantly segregate patients at greater risk for transfusion dependence. However, the presence of 2 or more aberrancies statistically defined two populations. Those possessing 0-1 blast aberrancy did not reach a median time to transfusion dependence, whereas those possessing 2+ aberrancies had a median time to transfusion dependence of 1.2 years (p=0.02). Additionally, when looking at neoplasia-specific blast aberrancies, pts with 0-1 aberrancy had a median time to transfusion of 4.7 years, compared to 2+ aberrancies, at 0.8 years (p=0.02). Figure 1 illustrates this finding.
Conclusion:
The determination of blast aberrancies by flow at time of MDS diagnosis may provide prognostic information in low/intermediate risk MDS patient and could help predict risk for early red blood cell transfusion dependence. Upfront risk stratification would be valuable information to plan follow-up for these patients, as well as treatment decision making including early initiation of ESAs.
Michaelis:Novartis: Consultancy; Celgene: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; JAZZ: Other: Data Safety Monitoring Board, uncompensated, Research Funding; BMS: Research Funding; Bioline: Research Funding; ASTEX: Research Funding; Janssen: Research Funding; Millenium: Research Funding; Macrogeneics: Research Funding; Pfizer: Equity Ownership, Research Funding; Incyte: Consultancy, Research Funding. Runaas:Agios: Honoraria; Blueprint Medicine: Honoraria. Atallah:Takeda: Consultancy, Research Funding; Pfizer: Consultancy; Jazz: Consultancy; Helsinn: Consultancy; Jazz: Consultancy; Novartis: Consultancy; Helsinn: Consultancy. Abedin:Actinium Pharmaceuticals: Research Funding; Pfizer Inc: Research Funding; Helsinn Healthcare: Research Funding; Agios: Honoraria; Jazz Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal