Introduction: Systemic Mastocytosis (SM) is a rare neoplasm of myeloid origin characterized by an accumulation of abnormal mast cells in the bone marrow, liver, spleen, and skin and has a molecular signature of the KITD816V mutation, detectable in >90% of patients. Clinical findings include cytopenias, liver-function abnormalities, hypoalbuminemia, weight loss, ascites, and osteolytic bone lesions due to infiltration of various organs by the malignant mast cells. The indolent and smoldering subtypes of SM can have a long natural history but aggressive systemic mastocytosis (aSM), SM with associated hematological neoplasm (SMHN), and mast cell leukemia (MCL) subtypes are associated with poor prognosis at a median survival of 3.5 years, 2 years and 6 months respectively. In April 2017, the FDA approved midostaurin, a multitargeted kinase inhibitor for the treatment of adults with aSM, SMHN and MCL at a dose of 100mg twice daily based on achievement of response rate endpoint noted in an open label trial of midostaurin (Gotlib et al, NEJM 2016). Pre-midostaurin, the treatment options for aSM, SMHN and MCL were imatinib, cladribine, interferon or allogeneic stem cell transplant (ASCT). The objective of this retrospective study was to assess the uptake and utilization of midostaurin in patients with aSM, SMHN and MCL.
Methods: Patients with at least 1 claim for midostaurin (index date) between April 1, 2017 and October 31, 2018 and who had at least 1 claim with a diagnosis of SM (ICD 10 code C96.2x or C94.3x) in the 6-month pre-index period were identified from Symphony Health's Integrated Dataverse (IDV), a large US claims database containing linked longitudinal prescription, medical, and hospital claims. The IDV contains claims for 280 million active unique patients representing over 73% of specialty prescriptions, 58% of medical claims, and 30% of hospital claims volume in the US. Patients included in the study cohort had a 6-month pre-index period with no claims with a diagnosis of SM, were at least 18 years old at initiation of midostaurin, and no evidence of participation in a clinical trial. Patient characteristics and treatment patterns were summarized using descriptive statistics. Median and 95% confidence interval (CI) for duration of midostaurin were calculated using Kaplan-Meier estimates.
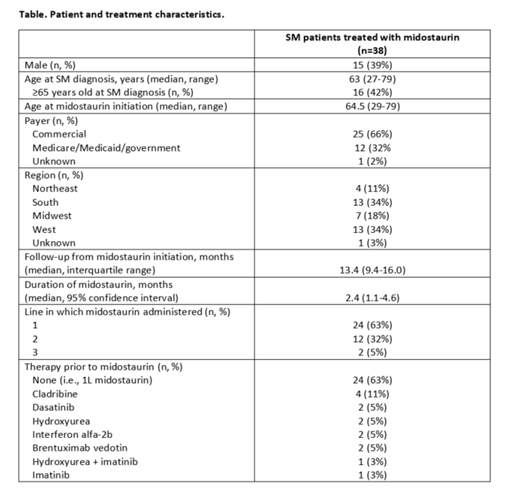
Results: Of the 38 patients with SM treated with midostaurin, 33 had a diagnosis of aSM and 5 had a diagnosis of MCL. The majority were female (61%) with a median age of 63 years (range 27-79) at diagnosis; 42% of these patients were 65 years or older (Table). The majority (66%) of patients had commercial insurance, and patients resided in all 4 US regions (34% South, 34% West, 18% Midwest, 11% Northeast). Nearly two-thirds (63%) of midostaurin use was in the first line setting, 32% was given in second line, and 5% was given in third line. Among the 14 patients with therapy prior to midostaurin, cladribine was most common (n=4, 29%), followed by hydroxyurea, interferon alfa-2b, brentuximab vedotin, and others (each n=2, 14%). Over two-thirds (68%) had an average daily dose of 200mg on the first fill, 5% treated at 100mg daily, and 26% with average daily doses <100mg. Two thirds (22 of 33) patients with aSM and 4 of 5 with MCL were prescribed the 200mg/day recommended dose. With a median follow-up of 13.4 months from midostaurin initiation, the median duration of midostaurin 2.4 months (95% CI 1.1-4.6).
Conclusions: In this retrospective claims based study, there is evidence of early uptake of midostaurin use in SM. Midostaurin was used in the first line setting in the majority of patients. The median duration of midostaurin treatment was short at 2.4 months especially when compared to a median treatment duration of 11.5 months in the pivotal trial. It is noteworthy that almost a third of patients were started on a lower than recommended dose which may compromise efficacy. The relatively short follow up may be another reason for the observed duration of response. Potential barriers to the appropriate use of midostaurin in SM such as lack of awareness, access, cost or delay in diagnosis due to its rarity will need further exploration. While the current claims-based dataset does not provide us the information, early, accurate diagnosis of SM is essential to timely utilization of midostaurin in the real-world setting. Further research and education may be needed to optimize the use of novel therapeutics in such orphan diseases.
Gajra:Cardinal Health: Employment. Klink:Cardinal Health: Employment. Chopra:Cardinal Health: Employment. Feinberg:Cardinal Health: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal