Background
Polycythemia vera (PV), characterized by erythrocytosis and JAK2 mutations, is associated with increased morbidity and mortality. Patients (pts) are at risk for thrombotic events and experience symptoms (sxs) that may impact quality of life. Data describing the clinical characteristics of pts with PV at the time of diagnosis (dx) are limited. The Prospective Observational Study of Patients with Polycythemia Vera in US Clinical Practices (REVEAL) is a multicenter, prospective, observational study designed to collect data on disease burden, clinical management, and pt-reported outcomes (PROs) of adult pts (aged ≥18) with PV. This analysis describes pt demographic and clinical characteristics, treatment patterns, and sxs of newly dxed pts with PV.
Methods
Pts enrolled in REVEAL who were dxed ≤6 months prior to enrollment were included in this analysis. Descriptive statistics were used to summarize pt demographic and clinical characteristics, tests at dx, and management patterns at dx. Sxs were assessed with a validated PRO instrument: the Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score (MPN-SAF TSS). Mean and standard deviation for individual sx scores and total sx score (TSS) at enrollment are reported.
Results
Of the 2510 pts enrolled in REVEAL, 321 dxed within 6 months of enrollment were included in this analysis. Median age at enrollment was 67.0 (range, 23.0-94.0) years, 54.8% were male, and most (74.5%) had high-risk disease (aged ≥60 years and/or history of thrombosis). The most common physician-reported symptoms at the time of presentation included fatigue (24.6%), pruritus (17.1%), insomnia (10.3%), and muscle aches/bone pain (9.7%). Of those pts reported to have undergone mutation testing at dx (n=201), 99.5% were JAK2V617F positive. A history of thrombosis was reported for 15.9% of pts (arterial, 8.1%; venous, 8.7%), and the most common relevant comorbidities were hypertension (58.6%), obesity (19.6%), and diabetes mellitus (15.9%). Of 188 pts with lab values within 1 month before dx, 95.2% had elevated (≥45%) hematocrit and 59.6% had elevated (>10 × 109/L) white blood cell counts. Most high-risk and low-risk pts were reported to have received phlebotomies within 6 months after dx (71.7% and 78.4%, respectively). A higher proportion of pts with high-risk disease initiated a pharmacologic therapy for PV compared with those who had low-risk disease (60.5% vs 23.9%); hydroxyurea (HU) was the most common cytoreductive therapy (n=151 [93.2%]). Most of these pts (n=137 [90.7%]) were still receiving HU 2 years after dx. Of those who initiated HU within 6 months of dx, 5.3% (n=8) received an alternative cytoreductive therapy following HU.
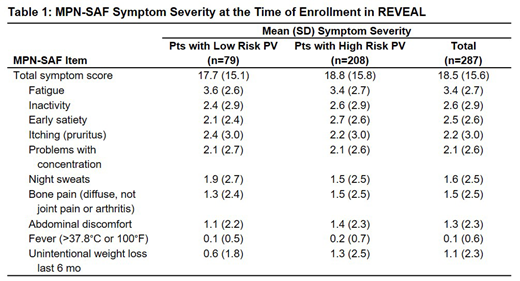
A total of 287 pts provided PRO data at enrollment (Table 1). The most common pt-reported sxs were fatigue (77.2%), early satiety (61.1%), and inactivity (58.0%); the mean (SD) MPN-SAF TSS was 18.5 (15.6). The sxs with the highest reported mean (SD) scores were fatigue (3.4 [2.7]), inactivity (2.6 [2.9]), and early satiety (2.5 [2.6]) and were largely similar between low-risk and high-risk pts.
Conclusions
Compared with all 2510 pts enrolled in REVEAL (Clin Lymphoma Myeloma Leuk 2018[18]12:788-95), this subgroup of 321 pts dxed within 6 months of enrollment was similar with respect to age and sex. However, in the newly dxed subgroup, a slightly lower proportion of pts (74.5%) had high-risk disease (77.3% of the full cohort). In the newly dxed subgroup, a higher proportion of pts (62.6%) underwent mutation testing (49.2% of all enrolled pts). The HU discontinuation rate early in the treatment course was approximately 9.3%, which is similar to the rate (6.3%) of HU discontinuation due to toxicity in patients with PV in a large, multicenter, MPN cohort (Am JHematol 2012[87]5:552-4). Sx burden in pts who completed the MPN-SAF TSS at enrollment was similar in the newly dxed pts (mean TSS, 18.5) and all pts enrolled in REVEAL (18.8; Clin Lymphoma Myeloma Leuk 2018[18]9:590-6); the most frequent pt-reported sxs (fatigue, early satiety, and inactivity) in this smaller pt subgroup were the same as those observed in the larger pt cohort. Overall, near the time of dx, a greater proportion of pts had low-risk disease. Despite this difference, the sx constellation and severity tends to be very similar to the larger cohort. It also appears that only a very small proportion of pts are unable to tolerate HU early on in the course of the disease.
Gerds:Sierra Oncology: Research Funding; Imago Biosciences: Research Funding; Incyte: Consultancy, Research Funding; CTI Biopharma: Consultancy, Research Funding; Pfizer: Consultancy; Celgene Corporation: Consultancy, Research Funding; Roche: Research Funding. Altomare:Novartis: Consultancy; Amgen: Consultancy; Incyte: Consultancy, Speakers Bureau; Rigel: Consultancy. Colucci:Incyte: Employment, Equity Ownership. Paranagama:Incyte: Employment, Equity Ownership. Burke:Roche/Genentech: Consultancy; Celgene: Consultancy; Gilead: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal