Introduction:
Tafasitamab (MOR208) is an Fc-enhanced, humanized, monoclonal antibody that targets CD19. L-MIND (NCT02399085) is an ongoing, open-label, single-arm, phase II study of Tafasitamab (TAFA) plus lenalidomide (LEN) in patients with relapsed/refractory (R/R) DLBCL ineligible for autologous stem cell transplantation (ASCT). A durable progression-free survival of 16 months in a poor-risk subgroup of patients treated with TAFA plus LEN was recently presented (Salles et al., 2019 ICML Meeting. Abstract 124). Treatment with CD19-targeted chimeric antigen receptor (CAR) T cell therapy has shown promising results for patients with R/R DLBCL after failure on ≥ 2 lines of systemic therapy (Neelapu S, et al. N Engl J Med 2017). Despite recent advances in anti-CD19 therapy, relapse remains frequent after each treatment modality. There is no clinical evidence to direct the sequencing of CAR T cell therapy after anti-CD19 therapy. Here, we present the first published case of an outcome after anti-CD19 antibody treatment TAFA/LEN as part of the L-MIND trial, followed by anti-CD19 CAR T therapy.
Case Presentation:
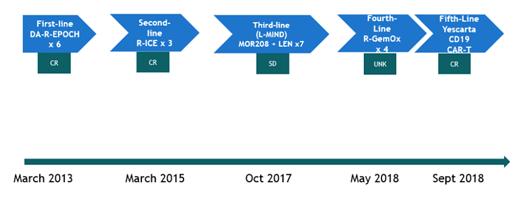
The patient is a 58 year old female who initially presented in March 2013 with an 8 cm mesenteric mass, workup revealed stage III germinal center B cell-like (GCB) DLBCL arising from follicular lymphoma, Ki-67 proliferation index 80%, and IGH/BCL2 fusion. The patient received 6 cycles of dose-adjusted R-EPOCH. Despite achieving a complete remission (CR) to this frontline therapy, the patient experienced disease relapse within two years. Subsequently, the patient received rituximab plus ifosfamide, carboplatin, and etoposide (RICE) chemotherapy, to which a second CR was achieved. The patient declined ASCT. Second relapse occurred approximately two years later. After meeting eligibility criteria, she was enrolled in the L-MIND trial in October 2017 and received TAFA plus LEN for 6 cycles (1 cycle = 28 days; TAFA 12mg/kg intravenously, weekly x 3 cycles, biweekly thereafter; LEN 25mg daily on days 1-21 of each cycle). TAFA/LEN was well tolerated. Stable disease was achieved with this investigational regimen, followed by progression in April 2018. Fourth-line treatment consisted of rituximab, gemcitabine, oxaliplatin, (R-GEM-OX) for 4 cycles, to which the patient had a partial response. Shortly thereafter, the patient received anti-CD19 CAR T therapy (axicabtagene ciloleucel [Yescarta]) in September 2018 (Figure 1). Treatment course was complicated by grade 2 cytokine release syndrome. A complete response was achieved one month after treatment, and has been sustained since then. As of this report (July 2019), patient remains without clinical evidence of relapse.
Discussion:
An important question to address in the era of targeted cellular therapy is: can the same tumor antigen be targeted with a different cancer immune therapy modality after disease progression following a previous therapy against that same antigen? In such a clinical scenario, there is concern for sustained antigen blockade from the prior line of therapy. In addition, there is concern for the selective pressures of prior therapy targeted against a specific antigen allowing for progression of a clone that does not express that antigen, rendering subsequent therapy against the same target ineffective (antigen escape). The half-life of TAFA is approximately 16 days, suggesting that it was eliminated prior to CAR T-cell infusion five months later. Although a biopsy was not done upon progression after TAFA, we assume that CD19 antigen escape did not account for the relapse, since the patient has achieved sustained remission with subsequent anti-CD19 CAR T cell therapy. Therefore, disease progression following treatment with anti-CD19 monoclonal antibody Tafasitamab may not preclude patients from anti-CD19 CAR T cell therapy, despite previously targeting the same antigen.
de Vos:Verastem: Consultancy; Portola Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal