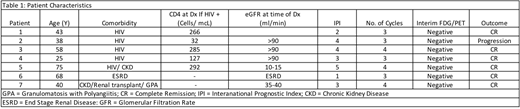
Background: While six cycles of chemotherapy is the standard curative approach in diffuse large B-cell lymphoma (DLBCL), patients with immunodeficiencies and/or organ compromise may experience significant toxicity with this duration of therapy. Hence, balancing the competing needs of optimal aggressive lymphoma therapy with tolerable treatment-related toxicity is a challenge in this population. Previously, abbreviated therapy with short-course etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin and dose-dense rituximab (SC-EPOCH-RR) - where most patients received 3 cycles of therapy - demonstrated high efficacy in HIV-associated DLBCL (Dunleavy et al. Blood 2010; 115:3017-24). 79% of patients received 3 cycles of therapy and overall 91% of patients achieved a complete response (CR). Here we retrospectively evaluate the outcome of patients with newly diagnosed DLBCL who had an underlying immunodeficiency and/or chronic renal failure and received SC-EPOCH-RR at our institution. Methods: We included patients who had a new diagnosis of DLBCL (including primary effusion lymphoma (PEL)) associated with immunodeficiency (HIV or post-organ transplant) and/or chronic renal failure. Therapy consisted of SC-EPOCH-RR (R was omitted in the patient with PEL) with intrathecal prophylaxis (as previously described). A total of three to four cycles of therapy was planned if an interim FDG-PET (following 2 cycles) was negative by Deauville criteria. A Deauville score of 1-3 was considered negative and 4-5 positive. In patients who were HIV positive, anti-retroviral therapy (ART) was continued as long as there were no significant ART-chemotherapy interactions. Results: Characteristics of 7 included patients are as follows: median age 43 (25-75); 5 (71%) were male; 6 (85.7%) had stage 3 or 4 disease; 6 (85.7%) had elevated LDH at diagnosis; 5(71.4%) had an International Prognostic index (IPI) score of 3-5; 5 (71%) were HIV positive and 3 (43%) had chronic renal failure (including 1 patient on chronic immunosuppression post-renal transplant for granulomatosis with polyangiitis). All had a diagnosis of DLBCL, 4 who were GCB and 2 non-GCB by the Hans algorithm, while 1 had extra-cavitary PEL. Two patients had c-MYC rearrangements by FISH. For HIV positive patients, the median CD4 count at diagnosis was 266 (32-292). No patient had CNS disease. All patients received 3 (4 patients) or 4 (3 patients) cycles of therapy. Responses were CR in all patients as assessed by end of therapy (EOT) FDG-PET. All patients had a negative interim PET by Deauville criteria (scores 1-3). One patient with HIV-associated DLBCL (CD4 count 32 at diagnosis and non-GCB subtype by immunohistochemistry) relapsed with CNS disease 4 weeks after achieving a negative EOT-PET and died from disease progression several weeks later. All other patients (6/7) are progression-free with a median follow-up time of 6 months. Conclusions: Albeit small numbers and short follow-up in our series, in a 'real-world' setting, abbreviated therapy was highly effective in patients with immunodeficiency-related DLBCL and/or in the setting of chronic renal failure. Using an interim PET guided approach, 3-4 cycles of therapy is highly effective and importantly limits toxicity in this population. Though the series was not large enough to accurately identify poor prognostic clinical and tumor factors, the patient with progression had a non-GCB tumor and was severely immunosuppressed, characteristics previously shown to be associated with adverse outcomes in this population. We continue to expand our series and follow-up. Additional studies are needed evaluating abbreviated therapy (SC-EPOCH-RR) in immunosuppressed and organ-compromised patients with aggressive B cell lymphomas for whom there is a paucity of experience and toxicity concerns limit delivery of standard, potentially curative therapy.
Smith:EUSA: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astra-Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Dunleavy:Pharmacyclics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal