Background
Currently available salvage chemotherapy regimens for patients (pts) with relapsed or refractory (RR) aggressive B-cell non-Hodgkin lymphoma (NHL) are mostly inpatient regimens and offer comparable response rates. Bendamustine has single agent activity in RR NHL. In vitro studies with Ofatumumab in NHL show more complement-dependent cytotoxicity than rituximab. We developed an outpatient salvage chemotherapy and replaced ifosfamide and rituximab in the R-ICE regimen with Bendamustine and Ofatumumab, respectively, in combination with Carboplatin and Etoposide (BOCE) in a phase I/II study. We previously reported the phase I study outcomes (Gaballa et al, ASCO 2014). Here, we report the final results of the BOCE phase I/II trial.
Methods
Patients were eligible if they had RR B-cell NHL. The phase I design was a standard 3+3 design using escalating doses of bendamustine (70, 90, 120 mg/m2 Days 1-2) combined with ofatumumab (cycle 1: 300mg Day 1, 1000mg Day 3; cycle 2 and 3: 1000mg Day 1), carboplatin (AUC 5 Day 2) and etoposide (100mg/m2 Days 1-3). No dose limiting toxicity (DLT) was observed in the phase I study and the recommended phase II dose (RP2D) of bendamustine was 120 mg/m2 Days 1-2.
Results
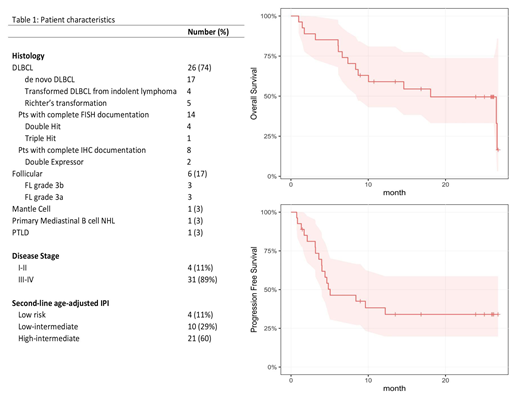
Thirty-six pts were enrolled (phase I, n=11; phase II, n=25; males, n=21; females, n=15). One patient withdrew consent prior to starting treatment and was not included in the analysis. Median age was 62 (range 41-78). Patients had relapsed (n=17, 49%) or refractory (n=18; 51%) NHL (table 1). Among DLBCL pts who had documented FISH (n=14), 4 were double hit (positive rearrangement for Myc and BCL-2), 1 was triple hit (positive rearrangement for Myc, BCL-2 and BCL-6). Of those pts with fully documented IHC (n=8), 2 patients were were double expressor. The number of median prior treatments was 1 (Range 1-5). There were no DLTs or treatment-related deaths. Grade III-IV toxicities included: neutropenia (71%), thrombocytopenia (66%), leukopenia (40%), neutropenic fever (31%), anemia (26%), lymphopenia (26%), hypophosphatemia (17%) and hyponatremia (11%). Eighteen serious adverse events occurred in 14 pts which included: infection (11%), neutropenic fever (9%), thrombocytopenia (6%), thromboembolic event (6%), dehydration (3%), hypercalcemia (3%), tumor lysis (3%), pre-syncope (3%) and GI bleeding (3%). Infusion-related reactions occurred in 40% of pts (all grade I-II).
Efficacy analysis was conducted on all phase II patients in addition to phase I patients who received the RP2D of bendamustine (n=27). The median-follow up was 23.9 months. The overall response rate was 70% (48% CR, 22% PR). Eight pts (30%) were refractory to treatment. The median progression-free survival (PFS) was 5.1 months and median overall-survival (OS) was 18.1 months. Fifteen pts died, all from disease progression. Of the 19 pts who responded, the median duration of response was 10.1 months. One patient failed stem-cell collection. Among all phase I and II patients, 12 patients subsequently underwent stem cell transplantation (SCT) (Autologous SCT, n=5; Allogeneic SCT, n=7). Autologous SCT was performed in RR DLBCL patients while allogeneic SCT was performed in: RR FL (n=4), transformed DLBCL (n=2), and Richter's transformation (n=1). In pts undergoing SCT, median PFS was not reached and median OS was 27.3 months.
Conclusions
BOCE is a safe and effective outpatient salvage chemotherapy regimen for pts with RR NHL. It yields favorable response rates compared to currently available salvage chemotherapy regimens and is an effective bridge to SCT. High grade toxicities were mostly hematologic. Patients who were successfully bridged to SCT had significantly better outcomes.
Pro:Seattle Genetics: Consultancy, Honoraria, Other: Travel Expenses, Research Funding; Takeda: Consultancy, Honoraria, Other: Travel Expenses; Celgene: Consultancy, Honoraria; Kyowa Hakka Kirin: Consultancy, Honoraria. Porcu:Innate Pharma: Honoraria, Other: Scientific Board, Research Funding; BeiGene: Other: Scientific Board, Research Funding; Incyte: Research Funding; Daiichi: Research Funding; Kyowa: Honoraria, Other: Scientific Board, Research Funding; ADCT: Research Funding; Spectrum: Consultancy; Viracta: Honoraria, Other: Scientific Board, Research Funding.
Bendamustine - alkylating agent with off-label use in relapse or refractory aggressive lymphomas Ofatumumab - monoclonal antibody which binds to CD20 resulting in complement-dependent cytotoxicity
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal