
Introduction:
Loncastuximab tesirine (Lonca) is an antibody-drug conjugate (ADC) comprising a humanized anti-CD19 antibody conjugated to a pyrrolobenzodiazepine dimer toxin. In a Phase 1, first-in-human study (ADCT-402-101), Lonca demonstrated single agent anti-tumor activity with manageable toxicity in patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL). Ibrutinib is a small-molecule inhibitor of Bruton's tyrosine kinase (BTK), a mediator of the B-cell-receptor signaling pathway implicated in the pathogenesis of B-cell cancers. BTK plays a crucial role in B-cell-receptor signaling, resulting in activation of pathways necessary for B-cell trafficking, chemotaxis, and adhesion. In vitro data show synergy between the two compounds, providing the rationale for studying them in combination.
Study Design and Methods:
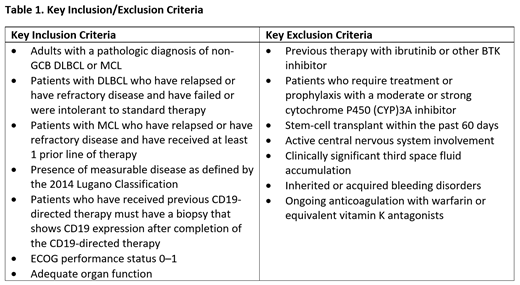
This is a Phase 1b (ADCT-402-103; NCT03684694), open-label, dose escalation (Part 1) and expansion (Part 2) trial of Lonca combined with ibrutinib in patients with R/R DLBCL or MCL. The key inclusion and exclusion criteria for this study are reported in Table 1. This trial will evaluate the safety and tolerability, preliminary anti-tumor activity, pharmacokinetics, pharmacodynamics, and immunogenicity of this combination. Patients will receive Lonca once every 3 weeks for a total of 2 doses, and ibrutinib daily for up to 1 year. Patients with a partial response or stable disease at the second disease evaluation may receive 2 additional doses of lonca. During Part 1, the dose of Lonca will be escalated using a classic 3+3 design. The dose of ibrutinib will be fixed. Part 2 will consist of up to 2 expansion cohorts, one for each of the DLBCL and MCL populations. All patients in Part 2 will receive the dose of Lonca determined in Part 1, with a fixed dose of ibrutinib. The trial opened in February 2019 and recruitment is ongoing.
Study sponsored by ADC Therapeutics SA, with the support of Pharmacyclics LLC, an AbbVie company, which supplies ibrutinib (http://clinicaltrials.gov/show/NCT03684694).
Ungar:ADC Therapeutics: Employment, Other: Stock options;equity interest. Dautaj:ADC Therapeutics: Employment, Other: Stock options. Wagner-Johnston:Jannsen: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal