INTRODUCTION: Brentuximab vedotin (Bv) is an antibody-drug conjugate with a CD30-directed antibody linked to the antitubulin agent monomethyl auristatin E (MMAE) which selectively induces apoptosis in CD30-positive malignant Reed-Sternberg cells. The use of Bv is thought to increase the possibility of cure and potentially avoid exposure to subsequent toxic therapies, therefore it is not surprising that Bv is becoming increasingly important in the treatment of Hodgkin lymphoma (HL). Bv has been studied in pediatric patients with relapsed/refractory HL and is currently under investigation as frontline therapy in high risk HL. However, little has been reported on the tolerability and treatment-related toxicity of Bv in the pediatric population. Here we report our experience with Bv in a small cohort of patients and report on the toxicities observed.
METHODS: We retrospectively evaluated the toxicity of Bv in children and adolescents with HL. Patients that received Bv either at the time of first diagnosis or relapse were included. Patient characteristics, treatment regimens including cumulative doses of Bv, toxicity and time to toxicity were collected.
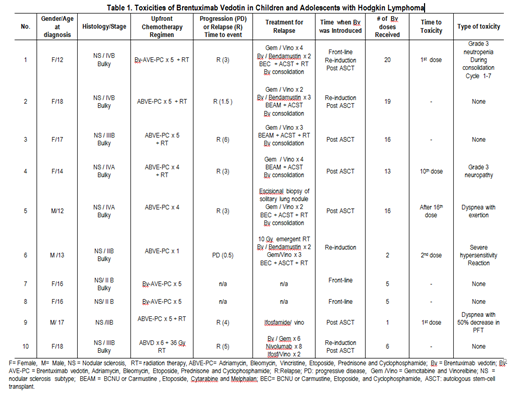
RESULTS: Ten patients with biopsy proven Hodgkin Lymphoma received treatment with Bv from 2015 to 2019 (Figure 1). Three patient received Bv as part of frontline therapy at the time of diagnosis. Of those, one received Bv for relapse therapy during re-induction and after autologous stem-cell transplant (ASCT). Seven patients received Bv for relapse/progressive disease. Of those 1 received it during re-induction therapy for relapse; 2 during re-induction and consolidation after ASCT; and 4 as consolidation after ASCT only. Five (50%) of the 10 patients developed Bv related toxicities. One patient experienced grade 3 neutropenia causing increased interval between doses. Two patients experienced dyspnea. One of them had mild dyspnea with exertion one month after he completed 16 doses of consolidative Bv. The other one had severe dyspnea with a decrease in pulmonary function (FCV 46, FEV1 < 50) when compared to baseline pulmonary function test (FCV 74, FEV1 of 70 at diagnosis with mediastinal mass). This patient did not receive any additional doses of Bv. Both of those patient received radiation therapy to the mediastinum during front-line treatment. One patient experienced grade 3 motor and sensory neuropathy requiring Bv to be discontinued at cycle 13. Neuropathy resolved four months after cessation of Bv. One patient had a grade 3 hypersensitivity reaction after second dose of Bv used as re-induction treatment for refractory HL. No clear association between the number of doses of Bv and toxicities was observed. Overall, we found severe neuropathy to be the most significant toxicity.
CONCLUSIONS: Our retrospective review highlights that the use of Bv either as frontline, re-induction therapy after relapse or as consolidative treatment following ASCT in children and adolescent patients, carries risk for toxicities. Some of these toxicities have significant impact in the quality of life such as neuropathy and dyspnea. Its use should be implemented with caution and monitored closely for toxicities. Ultimately, prospective studies are needed to evaluate and understand the acute and long term toxicities of the use of Bv in the treatment of HL either in the frontline, re-induction or consolidative therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal