Background: AITL, a histological nodal subtype of peripheral T-cell lymphoma (PTCL), is associated with a poor prognosis, particularly in the r/r setting, underscoring the need for effective salvage therapy. Small patient numbers coupled with a paucity of prospective studies evaluating treatment for r/r AITL provides little information for treatment decision-making. Pralatrexate is the first single agent approved globally in the United States for r/r PTCL based on the pivotal PROPEL trial, which was associated with an 8% (95% CI, 0 to 36%) objective response rate (ORR) in the thirteen r/r AITL patients (O'Connor et al, J Clin Oncol. 2011). Since then, two similar regulatory-mandated prospective trials in North Asian patients have been completed in China (Phase III, single-arm) and Japan (Phase I/II, single-arm). While the final follow-up for each of this study is still ongoing, the primary results were recently published, of which interestingly the former and latter AITL subgroups demonstrated an ORR of 55% (Hong et al, Target Oncol 2019) and 44% (Maruyama et al, Cancer Sci 2017) respectively.
Method: A post-hoc meta-analysis of patient level data pooled from these two trials conducted in China and Japan comprising of only r/r AITL patients was undertaken to provide insights about its efficacy and safety in this sub-population. r/r AITL was defined as having failed at least one prior line of therapy, with no upper limit on the number of prior therapies. Treatment-related adverse events of interest included in our analysis were oral mucositis/stomatitis and hematological toxicities (defined as any one of the following: anemia, decreased hemoglobin, granulocytopenia, leukopenia, lymphopenia, neutropenia/febrile neutropenia, pancytopenia, and thrombocytopenia). Cochran's Q-test and I2 were performed to investigate the existence of study heterogeneity.
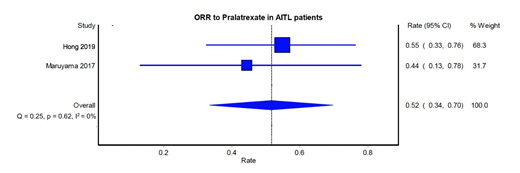
Results: From the total of 96 PTCL patients in the pivotal China (n=71) and Japan (n=25) studies, 29 patients were identified with AITL histology of which twenty were Chinese and nine Japanese. Median age at diagnosis was 62 years (range: 42-83 years). 48% (14 out of 29) of the study population were male. With a median of 2 prior lines of therapy, the ORR was 52% (15/29 patients; 95% CI, 34 to 70%). The median duration of response and overall survival were 4.5 months (136 days) and 9.7 months (295 days), respectively. Twenty-three patients (79.3%) experienced Grades 1-3 oral mucositis / mucositis / stomatitis and twenty-six (89.7%) experienced a hematological toxicity.
Conclusion: Though still of limited sample size, our results demonstrated antitumor activity with pralatrexate in r/r AITL patients, of which its safety and tolerability profile in this histological subtype corroborated with the general PTCL population as reported across the studies.
Zhu:Mundipharma: Research Funding. Yeoh:Mundipharma: Employment. Maeda:Astellas Pharma Inc.: Research Funding; Mundipharma Co Ltd.: Honoraria; Bristol-Myers Squibb: Honoraria, Research Funding; Kyowa Kirin Co. Ltd.: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal