Introdution: As a second-generation proteasome inhibitors (PIs), ixazomib has come to China for 1 years. PIs has been proved efficacy in patients with Waldenstrom macroglobulinemia (WM), a rare form of B-cell lymphoma.
Aims: To evaluated the clinical activity and safety profile of combining ixazomib and dexamethasone (ID) in patients with WM, both in those who previous had not received systematic treatment and in those with refractory or relapsed disease (RRWM).
Patients and Methods: The protocol therapy consisted of oral ixazomib, 4 mg, on days 1, 8, and 15, with oral dexamethasone, 10 mg, on days 1, 8, 15, and 22, every 4 weeks. Progression-free survival (PFS) was set as the primary end point. Response rates, sustained hematologic improvement from baseline, and safety were set as the key secondary end points. Responses were determined according to the 6th International Workshop WM response criteria. Adverse events (AEs) were reported by CTCAE v4.03. The data cut-off is 26 July 2019.
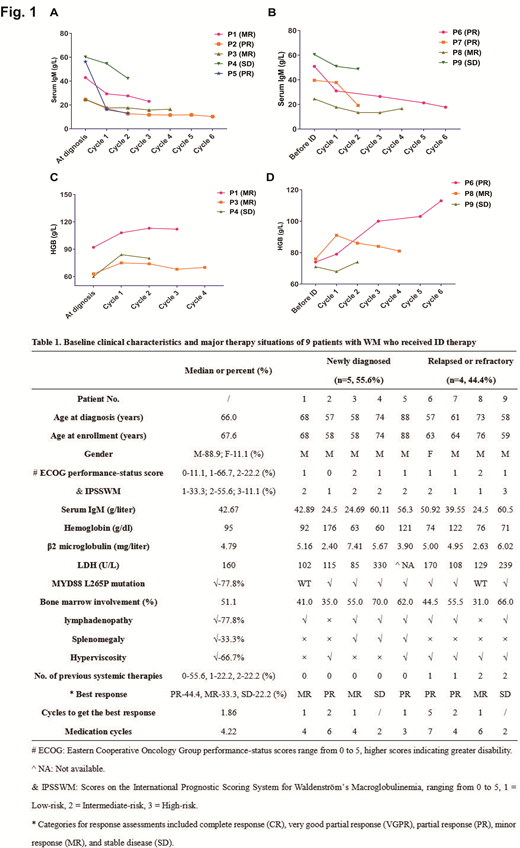
Results: Under the premise of respecting the patient preferences, 9 symptomatic patients with WM have been enrolled, in whom 5 patients were newly diagnosed (NDWM) receiving ID as primary therapy (PT) and the other 4 patients were with RRWM having been received previous systematic therapies. All patients were included in this analysis. The baseline clinical characteristics and major therapy situations are summarized in Table 1. The median reaction time was 4.14 weeks, which was 4.00 weeks in WM patients receiving ID as PT and 7.00 weeks in RR WM patients. After a median follow-up of 6.03 months, in NDWM patients, the overall response rate was 80.0%, and the major response rate (CR+VGPR+PR) was 40.0% (2/5). Patients with RRWM were equally responsive. The overall response rate was 75.0%, and the major response rate (CR+VGPR+PR) was even relatively higher, which was 50.0% (2/4). The median PFS was not reached. The ID regimen was well tolerated with 66.7% patients reporting no drug related AE. Grade 1 adverse events reported in 3 patients included diarrhea (1/9, 11.1%), thrombocytopenia (1/9, 11.1%), digestive disturbance(1/9, 11.1%). Shingles (grade 2) was reported in 1 patient. Serum IgM decreased from 42.66 g/l (median) at baseline to 21.02 g/l (median) in NDWM patients (Fig. 1A), from 43.87 g/l (median) at baseline to 25.59g/l (median) in RRWM patients (Fig. 1B). The median level of hemoglobin in patients with a hemoglobin of less than 115.0 g/l before using ID increased from 71.6 g/dl (median) at baseline to 87.3 g/l in NDWM patients (Fig. 1C), from 73.7 g/dl (median) at baseline to 89.3 g/l in RRWM patients (Fig. 1D). There have been 2 cases suffered disease progression after receiving ID regimen.
Conclusions: ID regimen offers a relatively effective and well tolerated choice for patients with WM.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal