Background:
Rituximab, the first FDA-approved anti-CD20 monoclonal antibody has dramatically improved outcomes for patients with CD20 positive lymphoproliferative disorders for 2 decades. Obinutuzumab was developed to potentiate activity and overcome resistance to rituximab. Clinical data suggest obinutuzumab is superior to rituximab in specific lymphoproliferative disorder, at the potential cost of increased toxicity, especially infections.
In order to better define the toxicity profile of obinutuzumab as opposed to rituximab, we conducted a systematic review and meta‐analysis of all randomized controlled trials comparing obinutuzumab with rituximab, either alone or combined with chemotherapy for any CD20 positive lymphoproliferative disorder, in order to assess safety.
Methods:
A comprehensive search was conducted until June 2019. Two reviewers appraised the quality of trials and extracted data. The Primary outcome was grade 3 to 4 infections; secondary outcomes included any adverse events, serious adverse events, and other grade 3 to 4 hematologic toxicity. Relative risks (RRs) with 95% confidence intervals (CIs) were estimated and pooled. A fixed‐effect model was used to pool data unless there was significant heterogeneity, in which case a random‐effects model was used.
Results:
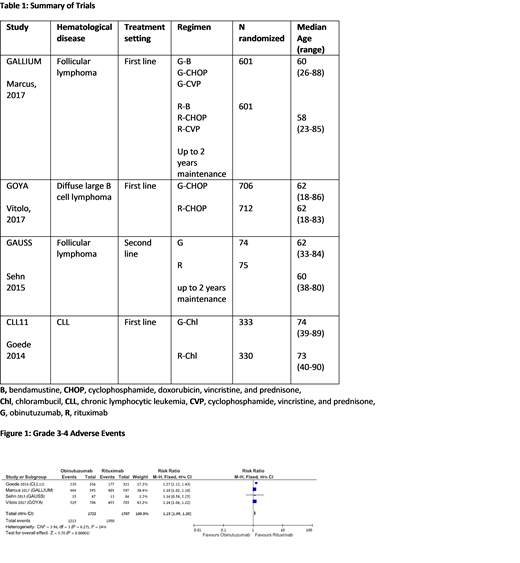
Our search yielded 4 randomized controlled trials conducted between the years 2009 and 2014, including 3429 patients. Median age of patients in the trials was 58 to 62 years. The trials included patients with follicular lymphoma (FL) (2 trials), chronic lymphocytic leukemia, (1 trial), and diffuse large B cell lymphoma (1 trial). All trials compared between obinutuzumab and rituximab therapy: in 3 trials the antibody was combined with chemotherapy and in 1 trial it was given alone. In 2 trials, which included FL patients, the antibody was given also as maintenance therapy for up to 2 years.
There was a statistically marginally increased rate of grade 3-4 infections (RR 1.17 [95% CI, 1.0‐1.36]) and febrile neutropenia (RR 1.23 [95% CI, 1.0‐1.5]) and a statistically significant increased rate of grade 3-4 adverse events (RR 1.15 [95% CI, 1.09‐1.2]) in the obinutuzumab arm vs rituximab. Also, the obinutuzumab arm showed increased risk of grade 3-4 thrombocytopenia (RR 2.8 [95% CI, 1.92‐4.06]), infusion related reactions (RR 2.8 [95% CI, 2.16‐3.64]) and cardiac events (RR 1.65 [95% CI, 1.11‐2.46]). There was no significant difference in grade 3-4 anemia and neutropenia rates, nor in the mortality rate at 36 months (RR 0.93 [95% CI, 0.78‐1.11]).
Conclusions:
Our systematic review and meta-analysis demonstrates that obinutuzumab has a more severe toxicity profile as compared to rituximab. As better clinical outcomes were reported with obinutuzumab in several CD20 positive lymphoproliferative disorders, but with more toxicity, clinicians are faced with a challenge upon deciding which therapy to choose for their patients.
Gurion:Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal