Introduction:
In children with sickle cell anemia (SCA) living in high-income settings, the routine use of transcranial Doppler (TCD) measurements, coupled with regular blood transfusion therapy for those with abnormal velocities (> 200 cm/sec) dramatically decreased the rate of strokes. Despite evidence of its beneficial effects in preventing strokes, regular blood transfusion therapy is not feasible for 99% of the children that are born with SCA living in low- and middle-income countries.
To address this gap in care, we tested the hypothesis that moderate fixed dose of hydroxyurea (20 mg/kg/day) for primary stroke prevention was feasible in a low-income setting, Kano, Nigeria. Specifically, we previously demonstrated that families were willing to enroll at a 92% recruitment rate and were adherent to the study protocol with no missed monthly research visits (603 of 603 visits). We have extended the trial for approximately five years to test the hypotheses that moderate fixed dose hydroxyurea will: 1) not result in excess incidence rate of severe adverse events (death or stroke) when compared to a group of children with SCA and TCD measurements less than 200 cm/sec; 2) decrease the incidence rate of strokes to the level of children receiving regular blood transfusion for primary stroke prevention in the STOP Trial. We report the final results of the primary Stroke Prevention Feasibility Trial in Nigeria (SPIN Trial (NCT01801423).
Methods:
At trial entry, all eligible participants were screened with non imaging TCD to determine increased stroke risk; defined as two independent measurements of time-averaged maximum velocity (TAMV) ≥ 200cm/sec in the middle cerebral artery (MCA) or one measurement >219cm/sec. Families were offered moderate fixed dose hydroxyurea (~20mg/kg/day). The comparison group included individuals with SCA that have a TCD measurement less than 200 cm/sec and agreed to be followed as routine medical care. Serious adverse events including death or stroke in the treatment and comparison groups were defined based on WHO criteria.
Results:
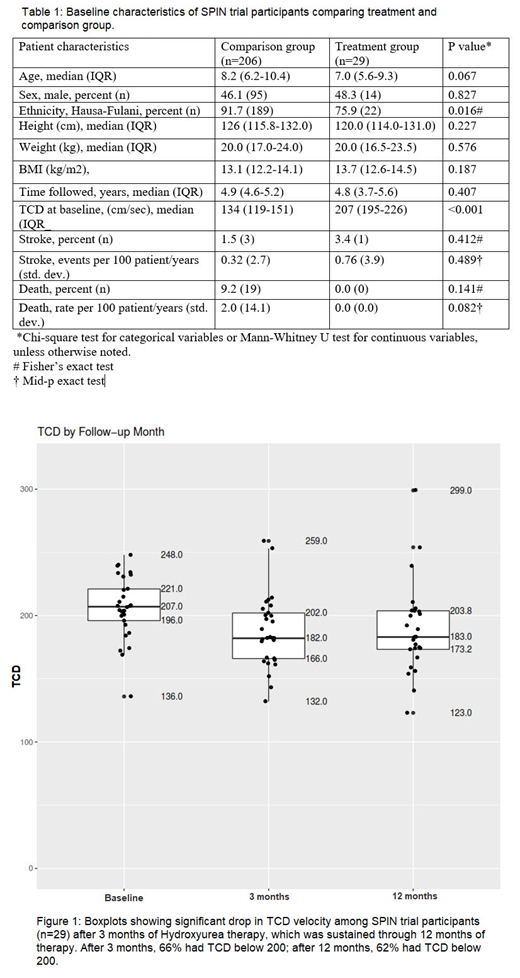
A total of 29 children treated with hydroxyurea for primary stroke prevention, and 206 children in the comparison group, were included. The median age for the treatment and comparison groups were 7.0 and 8.2 years, respectively. No statistically significant difference was observed in age, sex, ethnicity, height and weight of the treatment and comparison groups. Table 1 The median time on therapy (follow up time) was 4.9years (IQR- 4.60, 5.2). After 3 months of starting hydroxyurea therapy, 65.5% of the participants had a drop in TCD measurement to below 200cm/sec, which was sustained through 12months of therapy (61.5% had a TCD measurement below 200cm/sec at 12 months). Figure 1 The stroke incidence rate among the participants on hydroxyurea was 0.76 per 100 person-years (95% confidence interval 0.11 to 5.24), which was not significantly different from the incidence rate of 0.32 per 100 person-years (95% confidence interval 0.10 to 0.99) for participants in the comparison arm (P = 0.489), and significantly lower than the reported rate of stroke in the standard care group for the STOP study (10.7 per 100 person-years, total of 102 patient years).
A total of 20 deaths occurred, with no death in the treatment group. One death occurred in a child that was originally in the treatment group, but after the patient was withdrawn from the trial due to progressive renal disease unrelated to treatment. The death rate in the comparison group was 2.05 per 100 patient-years. There was no statistically significant difference in the death rate between treatment and comparison group (p = 0.082). The leading cause of death was suspected malaria occurring in 79% (15 of 19).
As expected, we found a statistically significant higher incidence rate of pain in the comparison group (30.04 per 100 person-years) compared to the treatment group (15.28 per 100 person-years) (P <0.001). No difference in incidence rates of anemia, infection, malaria and transfusion rates between the two groups.
Conclusion:
The extended results of the SPIN trial provide clear evidence that initial treatment with fixed moderate dose hydroxyurea (20 mg/kg/day) will prevent strokes in children with abnormal TCD measurements in a low-resource setting. The results of the extended SPIN trial have established the first evidence based stand care strategy for primary stroke prevention for children with SCA in Africa.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal